Meningeal CGRP-Prolactin Interaction Evokes Female-Specific Migraine Behavior
- PMID: 33749851
- PMCID: PMC8195469
- DOI: 10.1002/ana.26070
Meningeal CGRP-Prolactin Interaction Evokes Female-Specific Migraine Behavior
Abstract
Objective: Migraine is three times more common in women. CGRP plays a critical role in migraine pathology and causes female-specific behavioral responses upon meningeal application. These effects are likely mediated through interactions of CGRP with signaling systems specific to females. Prolactin (PRL) levels have been correlated with migraine attacks. Here, we explore a potential interaction between CGRP and PRL in the meninges.
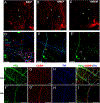
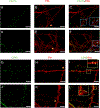
Methods: Prolactin, CGRP, and receptor antagonists CGRP8-37 or Δ1-9-G129R-hPRL were administered onto the dura of rodents followed by behavioral testing. Immunohistochemistry was used to examine PRL, CGRP and Prolactin receptor (Prlr) expression within the dura. Electrophysiology on cultured and back-labeled trigeminal ganglia (TG) neurons was used to assess PRL-induced excitability. Finally, the effects of PRL on evoked CGRP release from ex vivo dura were measured.
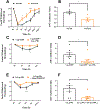
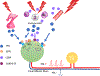
Results: We found that dural PRL produced sustained and long-lasting migraine-like behavior in cycling and ovariectomized female, but not male rodents. Prlr was expressed on dural afferent nerves in females with little-to-no presence in males. Consistent with this, PRL increased excitability only in female TG neurons innervating the dura and selectively sensitized CGRP release from female ex vivo dura. We demonstrate crosstalk between PRL and CGRP systems as CGRP8-37 decreases migraine-like responses to dural PRL. Reciprocally, Δ1-9-G129R-hPRL attenuates dural CGRP-induced migraine behaviors. Similarly, Prlr deletion from sensory neurons significantly reduced migraine-like responses to dural CGRP.
Interpretation: This CGRP-PRL interaction in the meninges is a mechanism by which these peptides could produce female-selective responses and increase the prevalence of migraine in women. ANN NEUROL 2021;89:1129-1144.
© 2021 American Neurological Association.
Conflict of interest statement
Potential Conflicts of Interest
Nothing to report.
Figures


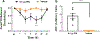
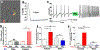
Comment in
-
Crosstalk between prolactin and CGRP signalling contributes to migraine in females.Nat Rev Neurol. 2021 May;17(5):261. doi: 10.1038/s41582-021-00491-y. Nat Rev Neurol. 2021. PMID: 33828293 No abstract available.
References
-
- Stewart WF, Shechter A, Rasmussen BK. Migraine prevalence. A review of population-based studies. Neurology 1994;44:S17–S23. - PubMed
-
- Buse DC, Loder EW, Gorman JA, et al. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American migraine prevalence and prevention (AMPP) study. Headache 2013; 53:1278–1299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

