Dermal fibroblast phagocytosis of apoptotic cells: A novel pathway for wound resolution
- PMID: 33749877
- PMCID: PMC8670562
- DOI: 10.1096/fj.202002078R
Dermal fibroblast phagocytosis of apoptotic cells: A novel pathway for wound resolution
Abstract
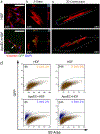
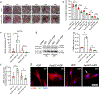
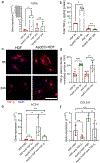
The effective clearance of apoptotic cells is an essential step in the resolution of healing wounds. In particular, blood vessel regression during wound resolution produces a significant number of apoptotic endothelial cells (ApoEC) that must be cleared. In considering the fate of ApoEC and the presence of fibroblasts during wound resolution, we hypothesized that fibroblasts might serve as phagocytes involved in endothelial cell removal. The current study investigated whether dermal fibroblasts engulf ApoEC, whether this uptake alters the phenotype of dermal fibroblasts, and the biological molecules involved. In both in vitro and in vivo studies, following ApoEC engulfment, fibroblasts acquired a pro-healing phenotype (increased cell migration, contractility, α-smooth muscle actin expression, and collagen deposition). In addition, fibroblast uptake of ApoEC was shown to be mediated in part by the milk fat globule-EGF factor 8 protein/integrin αv β5 pathway. Our study demonstrates a novel function of fibroblasts in the clearance of ApoEC and suggests that this capability has significant implications for tissue repair and fibrosis.
Keywords: apoptosis; endothelial cell; fibroblasts; milk fat globule-EGF factor 8 protein; phagocytosis; wound healing.
© 2021 Federation of American Societies for Experimental Biology.
Conflict of interest statement
CONFLICT OF INTEREST
The authors do not declare any financial interest. No ghostwriters were involved.
Figures





References
-
- Zeng R, Lin C, Lin Z, Chen H, Lu W, Lin C, and Li H Approaches to cutaneous wound healing: basics and future directions. Cell Tissue Res. 2018;374:217–232. - PubMed
-
- Zomer HD, and Trentin AG Skin wound healing in humans and mice: Challenges in translational research. J Dermatol Sci. 2018;90:3–12. - PubMed
-
- Dimmeler S, and Zeiher AM Endothelial cell apoptosis in angiogenesis and vessel regression. Circ. Res. 2000;87:434–439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

