Eosinophil ETosis-Mediated Release of Galectin-10 in Eosinophilic Granulomatosis With Polyangiitis
- PMID: 33750029
- PMCID: PMC8403105
- DOI: 10.1002/art.41727
Eosinophil ETosis-Mediated Release of Galectin-10 in Eosinophilic Granulomatosis With Polyangiitis
Abstract
Objective: Eosinophils are tissue-dwelling immune cells. Accumulating evidence indicates that a type of cell death termed ETosis is an important cell fate involved in the pathophysiology of inflammatory diseases. Although the critical role of eosinophils in eosinophilic granulomatosis with polyangiitis (EGPA; formerly Churg-Strauss syndrome) is well established, the presence of eosinophil ETosis (EETosis) is poorly understood. We undertook this study to better understand the characteristics of EETosis.
Methods: In vitro studies using blood-derived eosinophils were conducted to characterize EETosis. The occurrence of EETosis in tissues from patients with EGPA was studied by immunostaining and electron microscopy. Serum concentrations of eosinophil-derived proteins in healthy controls, patients with asthma, and EGPA patients with active disease or with disease in remission (n = 15 per group) were examined.
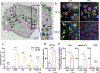
Results: EETosis was reliant on reactive oxygen species and peptidylarginine deiminase type 4-dependent histone citrullination, resulting in the cytolytic release of net-like eosinophil extracellular traps, free galectin-10, and membrane-bound intact granules. The signature of EETosis, including loss of cytoplasmic galectin-10 and deposition of granules, was observed in eosinophils infiltrating various tissues from EGPA patients. Serum eosinophil granule proteins and galectin-10 levels were increased in EGPA and positively correlated with disease activity as assessed by the Birmingham Vasculitis Activity Score (r = 0.8531, P < 0.0001 for galectin-10). When normalized to blood eosinophil counts, this correlation remained for galectin-10 (r = 0.7168, P < 0.0001) but not for granule proteins. Galectin-10 levels in active EGPA positively correlated with serum interleukin-5 levels.
Conclusion: Eosinophils infiltrating diseased tissues in EGPA undergo EETosis. Considering the exclusive expression and large pool of cytoplasmic galectin-10 in eosinophils, elevated serum galectin-10 levels in patients with EGPA might reflect the systemic occurrence of cytolytic EETosis.
© 2021, American College of Rheumatology.
Figures



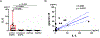
References
-
- Nagase H, Ueki S, Fujieda S. The roles of IL-5 and anti-IL-5 treatment in eosinophilic diseases: Asthma, eosinophilic granulomatosis with polyangiitis, and eosinophilic chronic rhinosinusitis. Allergol Int 2020;69:178–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

