Effects of maternal folic acid supplementation during the second and third trimesters of pregnancy on neurocognitive development in the child: an 11-year follow-up from a randomised controlled trial
- PMID: 33750355
- PMCID: PMC7945668
- DOI: 10.1186/s12916-021-01914-9
Effects of maternal folic acid supplementation during the second and third trimesters of pregnancy on neurocognitive development in the child: an 11-year follow-up from a randomised controlled trial
Abstract
Background: Maternal folic acid (FA) supplementation before and in early pregnancy prevents neural tube defects (NTD), but it is uncertain whether continuing FA after the first trimester has benefits on offspring health. We aimed to evaluate the effect of FA supplementation throughout pregnancy on cognitive performance and brain function in the child.
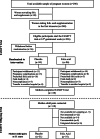
Methods: Follow-up investigation of 11-year-old children, residing in Northern Ireland, whose mothers had participated in a randomised trial of Folic Acid Supplementation in the Second and Third Trimesters (FASSTT) in pregnancy and received 400 μg/day FA or placebo from the 14th gestational week. Cognitive performance (Full Scale Intelligence Quotient, Verbal Comprehension, Working Memory, Perceptual Reasoning, and Processing Speed) was assessed using the Wechsler Intelligence Scale for Children. Neuronal function was assessed using magnetoencephalographic (MEG) brain imaging.
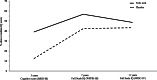
Results: Of 119 mother-child pairs in the FASSTT trial, 68 children were assessed for neurocognitive performance at 11-year follow-up (Dec 2017 to Nov 2018). Children of mothers randomised to FA compared with placebo scored significantly higher in two Processing Speed tests, i.e. symbol search (mean difference 2.9 points, 95% CI 0.3 to 5.5, p = 0.03) and cancellation (11.3 points, 2.5 to 20.1, p = 0.04), whereas the positive effect on Verbal Comprehension was significant in girls only (6.5 points, 1.2 to 11.8, p = 0.03). MEG assessment of neuronal responses to a language task showed increased power at the Beta (13-30 Hz, p = 0.01) and High Gamma (49-70 Hz, p = 0.04) bands in children from FA-supplemented mothers, suggesting more efficient semantic processing of language.
Conclusions: Continued FA supplementation in pregnancy beyond the early period currently recommended to prevent NTD can benefit neurocognitive development of the child. MEG provides a non-invasive tool in paediatric research to objectively assess functional brain activity in response to nutrition and other interventions.
Trial registration: ISRCTN ISRCTN19917787 . Registered on 15 May 2013.
Keywords: Child cognition; Magnetoencephalographic brain imaging; Neuronal function; Pregnancy; Prenatal folic acid; Randomised controlled trial; Wechsler Intelligence Scale for Children.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

