Cellular response of human apical papilla cells to calcium hydroxide and tricalcium silicate-based cements
- PMID: 33750358
- PMCID: PMC7941877
- DOI: 10.1186/s12903-021-01467-6
Cellular response of human apical papilla cells to calcium hydroxide and tricalcium silicate-based cements
Abstract
Background: This study aimed to evaluate the biological response of human apical papilla cells to different calcium hydroxide formulations and three tricalcium silicate-based materials.
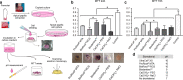
Methods: Primary cells were obtained from explants of young immature premolars. 20,000 cells adhered for 24 h over discs of Biodentine™, ProRoot®MTA, BioRoot®RCS and calcium hydroxide mixed either with sodium chloride 0.9%w/v or polyethylene glycol and UltraCal® were used to evaluate cell adhesion by scanning electron microscopy and cell viability by MTT assay.
Results: Cells adhered to ProRoot®MTA showed an increase of F-actin like protrusions, suggesting bioactivity. Cells adhered to UltraCal® show protrusion such as filopodia. On the contrary, cells adhered to BioRoot®RCS showed no signs of any cellular protrusion. Regarding viability between the materials, we found a higher percentage of viability in cells cultured over discs of Biodentine™ and ProRoot®MTA.
Conclusion: ProRoot®MTA and Biodentine™ exhibit a better cellular response of human apical papilla cells in vitro conditions compared to BioRoot® and calcium hydroxide diluted in sodium chloride.
Keywords: Apical papilla; Bioceramics; Calcium hydroxide.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Duggal M, Tong HJ, Al-Ansary M, Twati W, Day PF, Nazzal H. Interventions for the endodontic management of non-vital traumatised immature permanent anterior teeth in children and adolescents: a systematic review of the evidence and guidelines of the European Academy of Paediatric Dentistry. Eur Arch Paediatr Dent. 2017;18:139–151. doi: 10.1007/s40368-017-0289-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

