The impact of Pegylated liposomal doxorubicin in recurrent ovarian cancer: an updated meta-analysis of randomized clinical trials
- PMID: 33750444
- PMCID: PMC7945320
- DOI: 10.1186/s13048-021-00790-4
The impact of Pegylated liposomal doxorubicin in recurrent ovarian cancer: an updated meta-analysis of randomized clinical trials
Abstract
Background: Previous meta-analysis studies suggested that pegylated liposomal doxorubicin (PLD) may improve the survival rate of patients with recurrent ovarian cancer. The aim of the present meta-analysis, then, was to further update the role of PLD in the treatment of recurrent ovarian cancer.
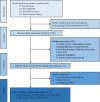
Methods: We performed a literature search using the electronic databases Medicine, EMBASE, Web of Science, and the Cochrane Library to 27 July 2020. We only restricted the randomized clinical trials. Study-specific hazard ratios and 95% confidence interval (HR/95% CI) and risk ratios and 95% confidence interval (RR/95% CI) were pooled using a random-effects model.
Results: Ten studies (12 trials) were included after screening 940 articles. We categorized the eligible studies into two groups: the doublet regimens (four trials, 1767 patients) showed that PLD plus carbo provided superior progression-free survival (PFS) (HR, 0.85; 95% CI, 0.74-0.97) and similar overall survival (OS) (HR, 1.00; 95% CI, 0.88-1.14) compared to paclitaxel (PAC) plus carboplatin (carbo). PLD plus carbo was associated with significantly more anemia and thrombocytopenia, and other side effects were well tolerated. The monotherapy regimens (eight trials, 1980 patients) showed that PLD possessed a similar PFS (HR, 1.02; 95% CI, 0.90-1.16) and OS (HR, 0.88; 95% CI, 0.77-1.01) relative to other monotherapies. PLD alone was also more associated with mucositis/stomatitis and hand-foot syndrome, while other side effects were well tolerated.
Conclusions: In platinum-sensitive recurrent ovarian cancer, PLD plus carbo was more effective than PAC plus carbo, while in platinum-resistant or -refractory recurrent ovarian cancer, PLD exhibited similar survival to other monotherapies. Regarding side effects, PLD plus carbo and mono chemotherapy were both well tolerated.
Keywords: Meta-analysis; Ovarian neoplasms; Overall survival; Pegylated liposomal doxorubicin; Progression-free survival.
Conflict of interest statement
All the authors declare no competing interests.
Figures




References
-
- Piao J, Lee EJ, Lee M. Association between pelvic inflammatory disease and risk of ovarian cancer: An updated meta-analysis. Gynecol Oncol. 2020;157:542-8. - PubMed
-
- Bookman MA, Okamoto A, Stuart G, Yanaihara N, Aoki D, Bacon M, et al. Harmonising clinical trials within the gynecologic cancer intergroup: consensus and unmet needs from the fifth ovarian cancer consensus conference. Ann Oncol. 2017;28:viii30–viii35. doi: 10.1093/annonc/mdx449. - DOI - PMC - PubMed
-
- Landrum LM, Brady WE, Armstrong DK, Moore KN, DiSilvestro PA, O'Malley DM, et al. A phase I trial of pegylated liposomal doxorubicin (PLD), carboplatin, bevacizumab and veliparib in recurrent, platinum-sensitive ovarian, primary peritoneal, and fallopian tube cancer: An NRG oncology/gynecologic oncology group study. Gynecol Oncol. 2016;140:204–209. doi: 10.1016/j.ygyno.2015.11.024. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

