National decision-making for the introduction of new vaccines: A systematic review, 2010-2020
- PMID: 33750592
- PMCID: PMC10370349
- DOI: 10.1016/j.vaccine.2021.02.059
National decision-making for the introduction of new vaccines: A systematic review, 2010-2020
Abstract
Background: Competing priorities make using a transparent and evidence-based approach important when deciding to recommend new vaccines. We conducted a literature review to document the processes and frameworks for national decision-making on new vaccine introductions and explored which key features have evolved since 2010.
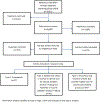
Methods: We searched literature published on policymaking related to vaccine introduction from March 2010 to August 2020 in six databases. We screened articles for eligibility with the following exclusion criteria: non-human or hypothetical vaccines, the sole focus on economic evaluation or decision to adopt rather than policy decision-making. We employed nine broad categories of criteria from the 2012 review for categorization and abstracted data on the country, income level, vaccine, and other relevant criteria.
Results: Of the 3808 unique references screened, 116 met eligibility criteria and were classified as: a) framework of vaccine adoption decision-making (27), b) studies that analyse empirical data on or examples of vaccine adoption decision-making (45), c) theoretical and empirical articles that provide insights into the vaccine policymaking process (44 + 17 already included in the previous categories). Commonly reported criteria for decision-making were the burden of disease; vaccine efficacy/effectiveness, safety; impact on health and non-health outcomes; economic evaluation and cost-effectiveness of alternative interventions. Programmatic and acceptability aspects were not as often considered. Most (50; 82%) of the 61 articles describing the process of vaccine introduction policymaking highlighted the role of country, regional, or global evidence-informed recommendations and a robust national governance as enabling factors for vaccine adoption.
Conclusions: The literature on vaccine adoption decision-making has expanded since 2010. We found that policymakers and expert advisory committee members (e.g., National Immunization Technical Advisory Group [NITAG]) increasingly value the interventions based on economic evaluations. The results of this review could guide discussions on evidence-informed immunization decision-making among country, sub-regional, and regional stakeholders.
Keywords: Evidence-based decision-making; Health system strengthening; Immunization programs; Infectious diseases; Systematic review; Vaccine policy.
Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- World Health Organisation. Vaccine introduction guidelines. Adding a vaccine to a national immunisation program: decision and implementation; 2005.
-
- World Health Organisation. Global Vaccine Action Plan 2011–2020; 2013, June 27 2018. Available: http://www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_2011...
-
- Duclos P. National Immunization Technical Advisory Groups (NITAGs): guidance for their establishment and strengthening. Vaccine 2010;28(Suppl 1):A18–25 (in eng). - PubMed
-
- Burchett HE, Mounier-Jack S, Griffiths UK, Mills AJ. National decision-making on adopting new vaccines: a systematic review. Health Policy Plann 2012;27 (Suppl 2):ii62–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous