Tumor-penetrating therapy for β5 integrin-rich pancreas cancer
- PMID: 33750829
- PMCID: PMC7943581
- DOI: 10.1038/s41467-021-21858-1
Tumor-penetrating therapy for β5 integrin-rich pancreas cancer
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is characterized by marked desmoplasia and drug resistance due, in part, to poor drug delivery to extravascular tumor tissue. Here, we report that carcinoma-associated fibroblasts (CAFs) induce β5 integrin expression in tumor cells in a TGF-β dependent manner, making them an efficient drug delivery target for the tumor-penetrating peptide iRGD. The capacity of iRGD to deliver conjugated and co-injected payloads is markedly suppressed when β5 integrins are knocked out in the tumor cells. Of note, β5 integrin knock-out in tumor cells leads to reduced disease burden and prolonged survival of the mice, demonstrating its contribution to PDAC progression. iRGD significantly potentiates co-injected chemotherapy in KPC mice with high β5 integrin expression and may be a powerful strategy to target an aggressive PDAC subpopulation.
Conflict of interest statement
K.N.S., V.R.K., T.T., and E.R. have ownership interest (including patents) in Cend Therapeutics Inc. K.N.S., T.T., and E.R. are also co-founders and stockholders of Cend Therapeutics Inc. E.R. is the chairman of the board of Cend Therapeutics Inc. No potential conflicts of interest were disclosed by the other authors.
Figures

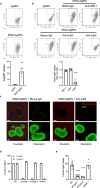

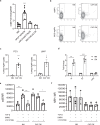


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

