Mathematical model for the thermal enhancement of radiation response: thermodynamic approach
- PMID: 33750833
- PMCID: PMC7970926
- DOI: 10.1038/s41598-021-84620-z
Mathematical model for the thermal enhancement of radiation response: thermodynamic approach
Abstract
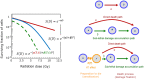
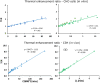
Radiotherapy can effectively kill malignant cells, but the doses required to cure cancer patients may inflict severe collateral damage to adjacent healthy tissues. Recent technological advances in the clinical application has revitalized hyperthermia treatment (HT) as an option to improve radiotherapy (RT) outcomes. Understanding the synergistic effect of simultaneous thermoradiotherapy via mathematical modelling is essential for treatment planning. We here propose a theoretical model in which the thermal enhancement ratio (TER) relates to the cell fraction being radiosensitised by the infliction of sublethal damage through HT. Further damage finally kills the cell or abrogates its proliferative capacity in a non-reversible process. We suggest the TER to be proportional to the energy invested in the sensitisation, which is modelled as a simple rate process. Assuming protein denaturation as the main driver of HT-induced sublethal damage and considering the temperature dependence of the heat capacity of cellular proteins, the sensitisation rates were found to depend exponentially on temperature; in agreement with previous empirical observations. Our findings point towards an improved definition of thermal dose in concordance with the thermodynamics of protein denaturation. Our predictions well reproduce experimental in vitro and in vivo data, explaining the thermal modulation of cellular radioresponse for simultaneous thermoradiotherapy.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Yagawa Y, Tanigawa K, Kobayashi Y, Yamamoto M. Cancer immunity and therapy using hyperthermia with immunotherapy, radiotherapy, chemotherapy, and surgery. J. Cancer Metastasis Treat. 2017;13:218–230. doi: 10.20517/2394-4722.2017.35. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

