Functional expression of complement factor I following AAV-mediated gene delivery in the retina of mice and human cells
- PMID: 33750925
- PMCID: PMC8149295
- DOI: 10.1038/s41434-021-00239-9
Functional expression of complement factor I following AAV-mediated gene delivery in the retina of mice and human cells
Abstract
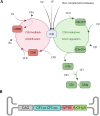
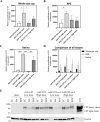
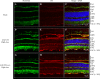
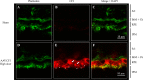
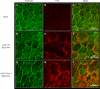
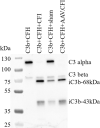
Dry age-related macular degeneration (AMD) is characterised by loss of central vision and currently has no approved medical treatment. Dysregulation of the complement system is thought to play an important role in disease pathology and supplementation of Complement Factor I (CFI), a key regulator of the complement system, has the potential to provide a treatment option for AMD. In this study, we demonstrate the generation of AAV constructs carrying the human CFI sequence and expression of CFI in cell lines and in the retina of C57BL/6 J mice. Four codon optimised constructs were compared to the most common human CFI sequence. All constructs expressed CFI protein; however, most codon optimised sequences resulted in significantly reduced CFI secretion compared to the non-optimised CFI sequence. In vivo expression analysis showed that CFI was predominantly expressed in the RPE and photoreceptors. Secreted protein in vitreous humour was demonstrated to be functionally active. The findings presented here have led to the formulation of an AAV-vectored gene therapy product currently being tested in a first-in-human clinical trial in subjects with geographic atrophy secondary to dry AMD (NCT03846193).
Conflict of interest statement
AKD and JPH are employed by Gyroscope Therapeutics Ltd, EO was employed by Gyroscope Therapeutics Ltd while engaged in the research project, PJL and REM are consultants to Gyroscope Therapeutics Ltd, MEM and ARB have no conflict of interest. AKD, JPH, PJL and REM have stock options in Gyroscope Therapeutics Limited.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

