Mammary gland adipocytes in lactation cycle, obesity and breast cancer
- PMID: 33751362
- PMCID: PMC8087566
- DOI: 10.1007/s11154-021-09633-5
Mammary gland adipocytes in lactation cycle, obesity and breast cancer
Abstract
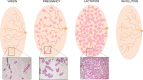
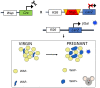
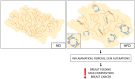
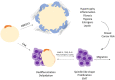
The mammary gland (MG) is an exocrine gland present in female mammals responsible for the production and secretion of milk during the process of lactation. It is mainly composed by epithelial cells and adipocytes. Among the features that make the MG unique there are 1) its highly plastic properties displayed during pregnancy, lactation and involution (all steps belonging to the lactation cycle) and 2) its requirement to grow in close association with adipocytes which are absolutely necessary to ensure MG's proper development at puberty and remodeling during the lactation cycle. Although MG adipocytes play such a critical role for the gland development, most of the studies have focused on its epithelial component only, leaving the role of the neighboring adipocytes largely unexplored. In this review we aim to describe evidences regarding MG's adipocytes role and properties in physiologic conditions (gland development and lactation cycle), obesity and breast cancer, emphasizing the existing gaps in the literature which deserve further investigation.
Keywords: Adipocytes; Breast cancer; Breastfeeding; Lactation; Mammary gland; Obesity.
Conflict of interest statement
Authors have nothing to disclose.
Figures



Similar articles
-
Remodeling of Murine Mammary Adipose Tissue during Pregnancy, Lactation, and Involution.J Mammary Gland Biol Neoplasia. 2019 Sep;24(3):207-212. doi: 10.1007/s10911-019-09434-2. Epub 2019 Sep 12. J Mammary Gland Biol Neoplasia. 2019. PMID: 31512027 Free PMC article. Review.
-
Natural and abrupt involution of the mammary gland affects differently the metabolic and health consequences of weaning.Life Sci. 2014 Apr 25;102(1):10-5. doi: 10.1016/j.lfs.2014.02.034. Epub 2014 Mar 7. Life Sci. 2014. PMID: 24607778 Review.
-
A cell transcriptomic profile provides insights into adipocytes of porcine mammary gland across development.J Anim Sci Biotechnol. 2023 Oct 8;14(1):126. doi: 10.1186/s40104-023-00926-0. J Anim Sci Biotechnol. 2023. PMID: 37805503 Free PMC article.
-
Mammary adipocyte flow cytometry as a tool to study mammary gland biology.FEBS Open Bio. 2023 Jul;13(7):1218-1227. doi: 10.1002/2211-5463.13620. Epub 2023 May 12. FEBS Open Bio. 2023. PMID: 37394996 Free PMC article.
-
Reversible transdifferentiation of secretory epithelial cells into adipocytes in the mammary gland.Proc Natl Acad Sci U S A. 2004 Nov 30;101(48):16801-6. doi: 10.1073/pnas.0407647101. Epub 2004 Nov 19. Proc Natl Acad Sci U S A. 2004. PMID: 15556998 Free PMC article.
Cited by
-
Early Life Stress, Brain Development, and Obesity Risk: Is Oxytocin the Missing Link?Cells. 2022 Feb 11;11(4):623. doi: 10.3390/cells11040623. Cells. 2022. PMID: 35203274 Free PMC article. Review.
-
Mature white adipocyte plasticity during mammary gland remodelling and cancer.Cell Insight. 2023 Aug 20;2(5):100123. doi: 10.1016/j.cellin.2023.100123. eCollection 2023 Oct. Cell Insight. 2023. PMID: 37771567 Free PMC article. Review.
-
Lack of fibro-inflammatory response in human mammary adipose tissue in obesity.Int J Obes (Lond). 2025 May;49(5):809-818. doi: 10.1038/s41366-024-01705-1. Epub 2024 Dec 29. Int J Obes (Lond). 2025. PMID: 39738492
-
Maternal Diet Quality in Pregnancy and Human Milk Extracellular Vesicle and Particle microRNA.Epigenet Rep. 2025;3(1):1-10. doi: 10.1080/28361512.2025.2508883. Epub 2025 May 30. Epigenet Rep. 2025. PMID: 40631350
-
Prenatal airborne polycyclic aromatic hydrocarbon exposure, altered regulation of peroxisome proliferator-activated receptor gamma (Ppar)γ, and links with mammary cancer.Environ Res. 2023 Aug 15;231(Pt 2):116213. doi: 10.1016/j.envres.2023.116213. Epub 2023 May 22. Environ Res. 2023. PMID: 37224940 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

