KY19382, a novel activator of Wnt/β-catenin signalling, promotes hair regrowth and hair follicle neogenesis
- PMID: 33751552
- PMCID: PMC8251890
- DOI: 10.1111/bph.15438
KY19382, a novel activator of Wnt/β-catenin signalling, promotes hair regrowth and hair follicle neogenesis
Abstract
Background and purpose: The promotion of hair regeneration and growth heavily depends on the activation of Wnt/β-catenin signalling in the hair follicle, including dermal papilla (DP). KY19382, one of the newly synthesized analogues of indirubin-3'-monoxime (I3O), was identified as a Wnt/β-catenin signalling activator via inhibition of the interaction between CXXC-type zinc finger protein 5 (CXXC5) and dishevelled (Dvl). Given the close relationship between the Wnt/β-catenin signalling and hair regeneration, we investigated the effect of KY19382 on hair regrowth and hair follicle neogenesis.
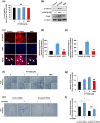
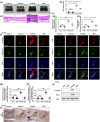
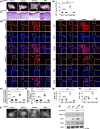
Experimental approach: In vitro hair induction effects of KY19382 were performed in human DP cells. The hair elongation effects of KY19382 were confirmed through the human hair follicle and vibrissa culture system. In vivo hair regeneration abilities of KY19382 were identified in three models: hair regrowth, wound-induced hair follicle neogenesis (WIHN) and hair patch assays using C57BL/6 mice. The hair regeneration abilities were analysed by immunoblotting, alkaline phosphatase (ALP) and immunohistochemical staining.
Key results: KY19382 activated Wnt/β-catenin signalling and elevated expression of ALP and the proliferation marker PCNA in DP cells. KY19382 also increased hair length in ex vivo-cultured mouse vibrissa and human hair follicles and induced hair regrowth in mice. Moreover, KY19382 significantly promoted the generation of de novo hair follicles as shown by WIHN and hair patch assays.
Conclusion and implications: These results indicate that KY19382 is a potential therapeutic drug that exhibits effective hair regeneration ability via activation of the Wnt/β-catenin signalling for alopecia treatments.
Keywords: CXXC5; GSK-3β; Wnt/β-catenin signalling; dermal papilla cells; neogenesis.
© 2021 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
K‐Y.C. is the CEO of CK Biotech. Inc. (Seoul, Korea), which has a licence to develop and use the compounds disclosed in the publication. The authors have no further conflicts of interest to declare.
Figures


References
-
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Sharman, J. L. , Southan, C. , Davies, J. A. , & CGTP Collaborators . (2019). The concise guide to PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 - DOI - PMC - PubMed
-
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , Cirino, G. , Docherty, J. R. , Giembycz, M. A. , Hoyer, D. , Insel, P. A. , Izzo, A. A. , Ji, Y. , MacEwan, D. J. , Mangum, J. , Wonnacott, S. , & Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology. British Journal of Pharmacology, 175(3), 407–411. 10.1111/bph.14112 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

