Dual Active Sites on Molybdenum/ZSM-5 Catalyst for Methane Dehydroaromatization: Insights from Solid-State NMR Spectroscopy
- PMID: 33751737
- PMCID: PMC8284829
- DOI: 10.1002/anie.202017074
Dual Active Sites on Molybdenum/ZSM-5 Catalyst for Methane Dehydroaromatization: Insights from Solid-State NMR Spectroscopy
Abstract
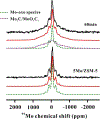
Methane dehydroaromatization (MDA) on Mo/ZSM-5 zeolite catalyst is promising for direct transformation of natural gas. Understanding the nature of active sites on Mo/ZSM-5 is a challenge for applications. Herein, using 1 H{95 Mo} double-resonance solid-state NMR spectroscopy, we identify proximate dual active sites on Mo/ZSM-5 catalyst by direct observation of internuclear spatial interaction between Brønsted acid site and Mo species in zeolite channels. The acidic proton-Mo spatial interaction is correlated with methane conversion and aromatics formation in the MDA process, an important factor in determining the catalyst activity and lifetime. The evolution of olefins and aromatics in Mo/ZSM-5 channels is monitored by detecting their host-guest interactions with both active Mo sites and Brønsted acid sites via 1 H{95 Mo} double-resonance and two-dimensional 1 H-1 H correlation NMR spectroscopy, revealing the intermediate role of olefins in hydrocarbon pool process during the MDA reaction.
Keywords: active sites; host-gust interactions; methane dehydroaromatization; solid state NMR; zeolites.
© 2021 Wiley-VCH GmbH.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures

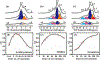


Similar articles
-
Active Ensembles in Methane Dehydroaromatization over Molybdenum/ZSM-5 Zeolite Identified by 2D 1 H-95 Mo Magic Angle Spinning Nuclear Magnetic Resonance Correlation Spectroscopy.Angew Chem Int Ed Engl. 2023 Aug 1;62(31):e202306133. doi: 10.1002/anie.202306133. Epub 2023 Jun 22. Angew Chem Int Ed Engl. 2023. PMID: 37261941
-
Confined Carbon Mediating Dehydroaromatization of Methane over Mo/ZSM-5.Angew Chem Int Ed Engl. 2018 Jan 22;57(4):1016-1020. doi: 10.1002/anie.201711098. Epub 2017 Dec 27. Angew Chem Int Ed Engl. 2018. PMID: 29181863 Free PMC article.
-
Understanding the Preparation and Reactivity of Mo/ZSM-5 Methane Dehydroaromatization Catalysts.Chemistry. 2022 Jan 24;28(5):e202103894. doi: 10.1002/chem.202103894. Epub 2021 Dec 16. Chemistry. 2022. PMID: 34822193 Free PMC article.
-
Insight into Three-Coordinate Aluminum Species on Ethanol-to-Olefin Conversion over ZSM-5 Zeolites.Angew Chem Int Ed Engl. 2019 Dec 9;58(50):18061-18068. doi: 10.1002/anie.201910987. Epub 2019 Oct 30. Angew Chem Int Ed Engl. 2019. PMID: 31592563 Review.
-
Reactivity, Selectivity, and Stability of Zeolite-Based Catalysts for Methane Dehydroaromatization.Adv Mater. 2020 Nov;32(44):e2002565. doi: 10.1002/adma.202002565. Epub 2020 Jul 12. Adv Mater. 2020. PMID: 32656906 Review.
Cited by
-
Emerging analytical methods to characterize zeolite-based materials.Natl Sci Rev. 2022 Mar 12;9(9):nwac047. doi: 10.1093/nsr/nwac047. eCollection 2022 Sep. Natl Sci Rev. 2022. PMID: 36128456 Free PMC article. Review.
-
Solid-State NMR Spectra of Protons and Quadrupolar Nuclei at 28.2 T: Resolving Signatures of Surface Sites with Fast Magic Angle Spinning.JACS Au. 2022 Oct 25;2(11):2460-2465. doi: 10.1021/jacsau.2c00510. eCollection 2022 Nov 28. JACS Au. 2022. PMID: 36465533 Free PMC article.
-
Direct conversion of methane to aromatics and hydrogen via a heterogeneous trimetallic synergistic catalyst.Nat Commun. 2024 Apr 16;15(1):3280. doi: 10.1038/s41467-024-47595-9. Nat Commun. 2024. PMID: 38627521 Free PMC article.
-
MoO3 Nanobelt-Modified HMCM-49 Zeolite with Enhanced Dispersion of Mo Species and Catalytic Performance for Methane Dehydro-Aromatization.Molecules. 2022 Jul 9;27(14):4404. doi: 10.3390/molecules27144404. Molecules. 2022. PMID: 35889276 Free PMC article.
-
Recent advances in solid-state NMR of zeolite catalysts.Natl Sci Rev. 2022 Aug 8;9(9):nwac155. doi: 10.1093/nsr/nwac155. eCollection 2022 Sep. Natl Sci Rev. 2022. PMID: 36131885 Free PMC article. Review.
References
-
- Wang L, Tao L, Xie M, Xu G, Huang J, Xu Y, Cata. Lett. 1993, 21, 35–41.
-
- Spivey JJ, Hutchings G, Chem. Soc. Rev. 2014, 43, 792–803. - PubMed
-
- Weckhuysen BM, Wang D, Rosynek MP, Lunsford JH, J. Catal. 1998, 175, 347–351.
-
- Weckhuysen BM, Wang D, Rosynek MP, Lunsford JH, J. Catal. 1998, 175, 338–346.
-
- Xu Y, Bao X, Lin L, J. Catal. 2003, 216, 386–395.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

