Itraconazole for COVID-19: preclinical studies and a proof-of-concept randomized clinical trial
- PMID: 33752127
- PMCID: PMC7979145
- DOI: 10.1016/j.ebiom.2021.103288
Itraconazole for COVID-19: preclinical studies and a proof-of-concept randomized clinical trial
Erratum in
-
Corrigendum to "itraconazole for COVID-19: Preclinical studies and a proof-of-concept randomized clinical trial Laurens".EBioMedicine. 2021 Jul;69:103454. doi: 10.1016/j.ebiom.2021.103454. Epub 2021 Jun 26. EBioMedicine. 2021. PMID: 34186486 Free PMC article. No abstract available.
Abstract
Background: The antifungal drug itraconazole exerts in vitro activity against SARS-CoV-2 in Vero and human Caco-2 cells. Preclinical and clinical studies are required to investigate if itraconazole is effective for the treatment and/or prevention of COVID-19.
Methods: Due to the initial absence of preclinical models, the effect of itraconazole was explored in a clinical, proof-of-concept, open-label, single-center study, in which hospitalized COVID-19 patients were randomly assigned to standard of care with or without itraconazole. Primary outcome was the cumulative score of the clinical status until day 15 based on the 7-point ordinal scale of the World Health Organization. In parallel, itraconazole was evaluated in a newly established hamster model of acute SARS-CoV-2 infection and transmission, as soon as the model was validated.
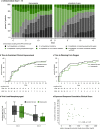
Findings: In the hamster acute infection model, itraconazole did not reduce viral load in lungs, stools or ileum, despite adequate plasma and lung drug concentrations. In the transmission model, itraconazole failed to prevent viral transmission. The clinical trial was prematurely discontinued after evaluation of the preclinical studies and because an interim analysis showed no signal for a more favorable outcome with itraconazole: mean cumulative score of the clinical status 49 vs 47, ratio of geometric means 1.01 (95% CI 0.85 to 1.19) for itraconazole vs standard of care.
Interpretation: Despite in vitro activity, itraconazole was not effective in a preclinical COVID-19 hamster model. This prompted the premature termination of the proof-of-concept clinical study.
Funding: KU Leuven, Research Foundation - Flanders (FWO), Horizon 2020, Bill and Melinda Gates Foundation.
Keywords: COVID-19; SARS-CoV-2; antivirals; drug repurposing; itraconazole.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Initial dug screening and discovery of the antiviral effect of itraconazole was done in collaboration with Johnson & Johnson, who also provided funding for this initial drug screen. Later in vitro drug screening was done independently from the company (e.g. antiviral activity of other azoles). Another manuscript in which an extensive panel of data is presented on the in vitro antiviral activity of itraconazole will be published together with authors from Johnson and Johnson. (Currently available as preprint [1]) Scientists from Johnson & Johnson performed drug measurements on hamster samples and provided guidance on the dosing regimens for the preclinical studies, but had no additional role in these experiments. The company had no role in the design, execution, analysis, publication or funding of the clinical trial. P Verhamme reports grants from KU Leuven during the conduct of the study and grants and personal fees for lectures and consultancy from Bayer Healthcare, Daiichi Sankyo, Pfizer, BMS, Bayer and Boehringer outside the submitted work, and personal fees for consultancy from Boehringer Ingelheim and Portola, outside the submitted work. Other authors have no conflict of interest to declare.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

