Remote intervention engagement and outcomes in the Clinical Trials in Organ Transplantation in Children consortium multisite trial
- PMID: 33752251
- PMCID: PMC8856090
- DOI: 10.1111/ajt.16567
Remote intervention engagement and outcomes in the Clinical Trials in Organ Transplantation in Children consortium multisite trial
Abstract
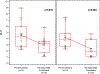
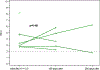
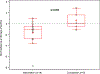
Remote interventions are increasingly used in transplant medicine but have rarely been rigorously evaluated. We investigated a remote intervention targeting immunosuppressant management in pediatric lung transplant recipients. Patients were recruited from a larger multisite trial if they had a Medication Level Variability Index (MLVI) ≥2.0, indicating worrisome tacrolimus level fluctuation. The manualized intervention included three weekly phone calls and regular follow-up calls. A comparison group included patients who met enrollment criteria after the subprotocol ended. Outcomes were defined before the intent-to-treat analysis. Feasibility was defined as ≥50% of participants completing the weekly calls. MLVI was compared pre- and 180 days postenrollment and between intervention and comparison groups. Of 18 eligible patients, 15 enrolled. Seven additional patients served as the comparison. Seventy-five percent of participants completed ≥3 weekly calls; average time on protocol was 257.7 days. Average intervention group MLVI was significantly lower (indicating improved blood level stability) at 180 days postenrollment (2.9 ± 1.29) compared with pre-enrollment (4.6 ± 2.10), p = .02. At 180 days, MLVI decreased by 1.6 points in the intervention group but increased by 0.6 in the comparison group (p = .054). Participants successfully engaged in a long-term remote intervention, and their medication blood levels stabilized. NCT02266888.
Keywords: clinical research/practice; compliance/adherence; immunosuppressant; pediatrics; social sciences.
© 2021 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM2 AI117870/AI/NIAID NIH HHS/United States
- F31HD096946/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- R01DK080740/DK/NIDDK NIH HHS/United States
- T32 AG066598/AG/NIA NIH HHS/United States
- U01AI077810/Division of Allergy, Immunology and Transplantation of the National Institutes of Health
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

