Non-compliance with pregnancy prevention recommendations for isotretinoin in Korea between 2019-2020
- PMID: 33752279
- PMCID: PMC7990996
- DOI: 10.5468/ogs.20247
Non-compliance with pregnancy prevention recommendations for isotretinoin in Korea between 2019-2020
Abstract
Objective: Isotretinoin is commonly prescribed worldwide despite its notorious teratogenicity. A risk management program (RMP) was introduced in Korea to prevent isotretinoin use during pregnancy. Here, we evaluate the compliance of Korean women with the recommendations of the RMP.
Methods: This prospective cohort study was conducted between April 2019 and June 2020. Thirty-six and 82 patients received the prescription before and after the introduction of RMP, respectively.
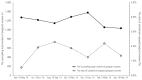
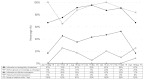
Results: There was a significant difference in the total number of days for which isotretinoin was prescribed before and after the RMP was introduced (68.8±100.9 and 28.0±26.1 days, respectively). However, 1.43% (120/8,394) of the total patients contacted by the teratology information services were exposed to isotretinoin on an average.
Conclusion: The proportion of patients exposed to isotretinoin did not change, and there was no significant change in compliance, with the implementation of the RMP during the study period. Further studies are needed to evaluate the effectiveness of the RMP in the long term.
Keywords: Abortion, induced; Congenital abnormalities; Isotretinoin; Pregnancy; Risk management.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Honein MA, Paulozzi LJ, Erickson JD. Continued occurrence of accutane-exposed pregnancies. Teratology. 2001;64:142–7. - PubMed
-
- Azoulay L, Oraichi D, Bérard A. Patterns and utilization of isotretinoin for acne from 1984 to 2003: is there need for concern? Eur J Clin Pharmacol. 2006;62:667–74. - PubMed
-
- Lammer EJ, Chen DT, Hoar RM, Agnish ND, Benke PJ, Braun JT, et al. Retinoic acid embryopathy. N Engl J Med. 1985;313:837–41. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

