High-Sensitivity Cardiac Troponin on Presentation to Rule Out Myocardial Infarction: A Stepped-Wedge Cluster Randomized Controlled Trial
- PMID: 33752439
- PMCID: PMC8177493
- DOI: 10.1161/CIRCULATIONAHA.120.052380
High-Sensitivity Cardiac Troponin on Presentation to Rule Out Myocardial Infarction: A Stepped-Wedge Cluster Randomized Controlled Trial
Abstract
Background: High-sensitivity cardiac troponin assays enable myocardial infarction to be ruled out earlier, but the safety and efficacy of this approach is uncertain. We investigated whether an early rule-out pathway is safe and effective for patients with suspected acute coronary syndrome.
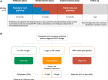
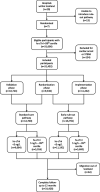
Methods: We performed a stepped-wedge cluster randomized controlled trial in the emergency departments of 7 acute care hospitals in Scotland. Consecutive patients presenting with suspected acute coronary syndrome between December 2014 and December 2016 were included. Sites were randomized to implement an early rule-out pathway where myocardial infarction was excluded if high-sensitivity cardiac troponin I concentrations were <5 ng/L at presentation. During a previous validation phase, myocardial infarction was ruled out when troponin concentrations were <99th percentile at 6 to 12 hours after symptom onset. The coprimary outcome was length of stay (efficacy) and myocardial infarction or cardiac death after discharge at 30 days (safety). Patients were followed for 1 year to evaluate safety and other secondary outcomes.
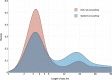
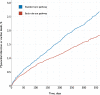
Results: We enrolled 31 492 patients (59±17 years of age [mean±SD]; 45% women) with troponin concentrations <99th percentile at presentation. Length of stay was reduced from 10.1±4.1 to 6.8±3.9 hours (adjusted geometric mean ratio, 0.78 [95% CI, 0.73-0.83]; P<0.001) after implementation and the proportion of patients discharged increased from 50% to 71% (adjusted odds ratio, 1.59 [95% CI, 1.45-1.75]). Noninferiority was not demonstrated for the 30-day safety outcome (upper limit of 1-sided 95% CI for adjusted risk difference, 0.70% [noninferiority margin 0.50%]; P=0.068), but the observed differences favored the early rule-out pathway (0.4% [57/14 700] versus 0.3% [56/16 792]). At 1 year, the safety outcome occurred in 2.7% (396/14 700) and 1.8% (307/16 792) of patients before and after implementation (adjusted odds ratio, 1.02 [95% CI, 0.74-1.40]; P=0.894), and there were no differences in hospital reattendance or all-cause mortality.
Conclusions: Implementation of an early rule-out pathway for myocardial infarction reduced length of stay and hospital admission. Although noninferiority for the safety outcome was not demonstrated at 30 days, there was no increase in cardiac events at 1 year. Adoption of this pathway would have major benefits for patients and health care providers. Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT03005158.
Keywords: biomarkers; chest pain; myocardial infarction; randomized controlled trial; troponin.
Figures
Comment in
-
Raising the Bar for Clinical Cardiac Troponin Research Studies and Implementation Science.Circulation. 2021 Jun 8;143(23):2225-2228. doi: 10.1161/CIRCULATIONAHA.121.054926. Epub 2021 Jun 7. Circulation. 2021. PMID: 34097442 No abstract available.
References
-
- Hollander JE, Than M, Mueller C. State-of-the-art evaluation of emergency department patients presenting with potential acute coronary syndromes. Circulation. 2016;134:547–564. doi: 10.1161/CIRCULATIONAHA.116.021886 - PubMed
-
- Anderson JL, Morrow DA. Acute myocardial infarction. N Engl J Med. 2017;376:2053–2064. doi: 10.1056/NEJMra1606915 - PubMed
-
- Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, et al. ; Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058 - PubMed
-
- Apple FS, Collinson PO; IFCC Task Force on Clinical Applications of Cardiac Biomarkers. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem. 2012;58:54–61. doi: 10.1373/clinchem.2011.165795 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- RE/18/6134217/BHF_/British Heart Foundation/United Kingdom
- CH/F/21/90010/BHF_/British Heart Foundation/United Kingdom
- FS/16/14/32023/BHF_/British Heart Foundation/United Kingdom
- MR/W000598/1/MRC_/Medical Research Council/United Kingdom
- SP/12/10/29922/BHF_/British Heart Foundation/United Kingdom
- RG/20/10/34966/BHF_/British Heart Foundation/United Kingdom
- RE/18/5/34216/BHF_/British Heart Foundation/United Kingdom
- WT103782AIA/WT_/Wellcome Trust/United Kingdom
- MR/V007017/1/MRC_/Medical Research Council/United Kingdom
- CH/09/002/26360/BHF_/British Heart Foundation/United Kingdom
- FS/18/25/33454/BHF_/British Heart Foundation/United Kingdom
- FS/19/17/34172/BHF_/British Heart Foundation/United Kingdom
- PG/15/51/31596/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

