Hypoxia-Inducible Factor 2a Expression Is Positively Correlated With Gleason Score in Prostate Cancer
- PMID: 33752529
- PMCID: PMC8093782
- DOI: 10.1177/1533033821990010
Hypoxia-Inducible Factor 2a Expression Is Positively Correlated With Gleason Score in Prostate Cancer
Abstract
Background: One of the main factors in response to hypoxia in the tumor microenvironment is the hypoxia-inducible factor (HIF) pathway. Although its role in other solid tumors, particularly renal cell carcinoma, has been sufficiently elucidated, it remains elusive in prostate cancer. The aim of the present study was to investigate the expression of main proteins involved in this pathway and determine the correlation of the results with clinicopathological outcomes of patients with prostate cancer.
Methods: The immunohistochemical expression of HIF-1a, HIF-2a and their regulators, prolyl hydroxylase domain (PHD)1, PHD2 and PHD3 and factor inhibiting HIF (FIH), was assessed on a tissue microarray. This was constructed from radical prostatectomy specimens, involving both tumor and corresponding adjacent non-tumoral prostate tissues from 50 patients with localized or locally advanced prostate cancer.
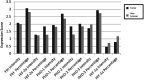
Results: In comparison with non-tumoral adjacent tissue, HIF-1a exhibited an equal or lower expression in 86% of the specimens (P = 0.017), while HIF-2a was overexpressed in 52% (P = 0.032) of the cases. HIF-1a protein expression was correlated with HIF-2a (P < 0.001), FIH (P = 0.004), PHD1 (P < 0.001), PHD2 (P < 0.001) and PHD3 (P = 0.035). HIF-2a expression was positively correlated with Gleason score (P = 0.017) and International Society of Urological Pathologists (ISUP) grade group (P = 0.022).
Conclusions: The findings of the present study suggest a key role for HIF-2a in prostate cancer, as HIF-2a expression was found to be correlated with Gleason score and ISUP grade of the patients. However, further studies are required to validate these results and investigate the potential value of HIF-2a as a therapeutic target in prostate cancer.
Keywords: FIH; Gleason score; HIF; hypoxia; prostate cancer.
Conflict of interest statement
Figures



References
-
- Mabjeesh NJ, Amir S. Hypoxia-inducible factor (HIF) in human tumorigenesis. Histol Histopathol. 2007;22(5):559–572. doi:10.14670/HH-22.559 - PubMed
-
- Kimbro KS, Simons JW. Hypoxia-inducible factor-1 in human breast and prostate cancer. Endocr Relat Cancer. 2006;13(3):739–749. doi:10.1677/erc.1.00728 - PubMed
-
- Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol. 2006;59(1):15–26. doi:10.1016/j.critrevonc.2005.12.003 - PubMed
-
- Boddy JL, Fox SB, Han C, et al. The androgen receptor is significantly associated with vascular endothelial growth factor and hypoxia sensing via hypoxia-inducible factors HIF-1a, HIF-2a, and the prolyl hydroxylases in human prostate cancer. Clin Cancer Res. 2005;11(21):7658–7663. doi:10.1158/1078-0432.CCR-05-0460 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

