SCN11A gene deletion causes sensorineural hearing loss by impairing the ribbon synapses and auditory nerves
- PMID: 33752606
- PMCID: PMC7986359
- DOI: 10.1186/s12868-021-00613-8
SCN11A gene deletion causes sensorineural hearing loss by impairing the ribbon synapses and auditory nerves
Abstract
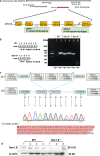
Background: The SCN11A gene, encoded Nav1.9 TTX resistant sodium channels, is a main effector in peripheral inflammation related pain in nociceptive neurons. The role of SCN11A gene in the auditory system has not been well characterized. We therefore examined the expression of SCN11A in the murine cochlea, the morphological and physiological features of Nav1.9 knockout (KO) ICR mice.
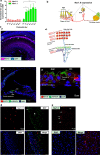
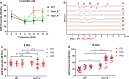
Results: Nav1.9 expression was found in the primary afferent endings beneath the inner hair cells (IHCs). The relative quantitative expression of Nav1.9 mRNA in modiolus of wild-type (WT) mice remains unchanged from P0 to P60. The number of presynaptic CtBP2 puncta in Nav1.9 KO mice was significantly lower than WT. In addition, the number of SGNs in Nav1.9 KO mice was also less than WT in the basal turn, but not in the apical and middle turns. There was no lesion in the somas and stereocilia of hair cells in Nav1.9 KO mice. Furthermore, Nav1.9 KO mice showed higher and progressive elevated ABR threshold at 16 kHz, and a significant increase in CAP thresholds.
Conclusions: These data suggest a role of Nav1.9 in regulating the function of ribbon synapses and the auditory nerves. The impairment induced by Nav1.9 gene deletion mimics the characters of cochlear synaptopathy.
Keywords: Expression; Nav1.9 knockout; Progressive hearing loss; Ribbon synapse; SGN; Synaptopathy; TTX resistant sodium channels.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Loss of inner hair cell ribbon synapses and auditory nerve fiber regression in Cldn14 knockout mice.Hear Res. 2020 Jun;391:107950. doi: 10.1016/j.heares.2020.107950. Epub 2020 Mar 16. Hear Res. 2020. PMID: 32251970
-
SMAD4 Defect Causes Auditory Neuropathy Via Specialized Disruption of Cochlear Ribbon Synapses in Mice.Mol Neurobiol. 2016 Oct;53(8):5679-91. doi: 10.1007/s12035-015-9454-1. Epub 2015 Oct 21. Mol Neurobiol. 2016. PMID: 26491026
-
Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms.Hear Res. 2017 Jun;349:138-147. doi: 10.1016/j.heares.2017.01.003. Epub 2017 Jan 10. Hear Res. 2017. PMID: 28087419 Free PMC article. Review.
-
Vesicular Glutamatergic Transmission in Noise-Induced Loss and Repair of Cochlear Ribbon Synapses.J Neurosci. 2019 Jun 5;39(23):4434-4447. doi: 10.1523/JNEUROSCI.2228-18.2019. Epub 2019 Mar 29. J Neurosci. 2019. PMID: 30926748 Free PMC article.
-
Auditory neuropathy--neural and synaptic mechanisms.Nat Rev Neurol. 2016 Mar;12(3):135-49. doi: 10.1038/nrneurol.2016.10. Epub 2016 Feb 19. Nat Rev Neurol. 2016. PMID: 26891769 Review.
Cited by
-
Expression and Physiology of Voltage-Gated Sodium Channels in Developing Human Inner Ear.Front Neurosci. 2021 Oct 25;15:733291. doi: 10.3389/fnins.2021.733291. eCollection 2021. Front Neurosci. 2021. PMID: 34759790 Free PMC article.
-
ZBTB20 is essential for cochlear maturation and hearing in mice.Proc Natl Acad Sci U S A. 2023 Jun 13;120(24):e2220867120. doi: 10.1073/pnas.2220867120. Epub 2023 Jun 6. Proc Natl Acad Sci U S A. 2023. PMID: 37279265 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

