Culture-dependent and culture-independent characterization of bacterial community diversity in different types of sandy lands: the case of Minqin County, China
- PMID: 33752616
- PMCID: PMC7986352
- DOI: 10.1186/s12866-021-02150-0
Culture-dependent and culture-independent characterization of bacterial community diversity in different types of sandy lands: the case of Minqin County, China
Abstract
Background: Minqin is suffering from a serious desertification, whereas the knowledge about its bacterial community is limited. Herein, based on Nitraria tangutorum and Haloxylon ammodendron from Minqin, the bacterial community diversities in fixed sandy land, semi-fixed sandy land and shifting sandy land were investigated by combining with culture-dependent and culture-independent methods.
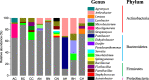
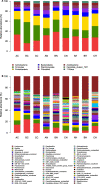
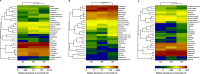
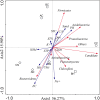
Results: Minqin stressed with high salinity and poor nutrition is an oligotrophic environment. Bacterial community in Minqin was shaped primarily by the presence of host plants, whereas the type of plant and sandy land had no marked effect on those, which displayed a better survival in the rhizospheres of N. tangutorum and H. ammodendron. The dominant groups at phyla level were Actinobacteria, Firmicutes, Proteobacteria, Bacteroidetes, Planctomycetes, Chloroflexi, Acidobacteria and Candidate_division_TM7. The abundance of Firmicutes with ability of desiccation-tolerance was significantly higher in harsh environment, whereas Bacteroidetes were mainly distributed in areas with high nutrient content. The abundances of Proteobacteria and Bacteroidetes were relatively high in the rhizospheres of N. tangutorum and H. ammodendron, which had more plant-growth promoting rhizobacteria. A large number of Actinobacteria were detected, of which the most abundant genus was Streptomyces. The physicochemical factors related to the diversity and distribution of the bacterial community were comprehensively analyzed, such as pH, electrical conductivity, soil organic matter, C/N and sand, and the results indicated that Minqin was more suitable for the growth of N. tangutorum, which should be one of most important sand-fixing plants in Minqin.
Conclusions: The bacterial community diversities in different types of sandy lands of Minqin were comprehensively and systematically investigated by culture-dependent and culture-independent approaches, which has a great significance in maintaining/restoring biological diversity.
Keywords: Bacteria community; Culture-dependent method; Culture-independent method; Desertification; Minqin Desert; Rhizosphere; Sandy land.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
The Composition and Diversity of the Rhizosphere Bacterial Community of Ammodendron bifolium Growing in the Takeermohuer Desert Are Different from Those in the Nonrhizosphere.Microb Ecol. 2023 Nov 27;87(1):2. doi: 10.1007/s00248-023-02320-9. Microb Ecol. 2023. PMID: 38008827
-
Composition and Distribution Characteristics of Rhizosphere Bacterial Community of Ammodendron bifolium Growing in Takeermohuer Desert Are Different from Those in Non-rhizosphere.Microb Ecol. 2023 Nov;86(4):2461-2476. doi: 10.1007/s00248-023-02252-4. Epub 2023 Jun 10. Microb Ecol. 2023. PMID: 37301781
-
[Prediction of Soil Bacterial Community Structure and Function in Minqin Desert-oasis Ecotone Artificial Haloxylon ammodendron Forest].Huan Jing Ke Xue. 2024 Jan 8;45(1):508-519. doi: 10.13227/j.hjkx.202302155. Huan Jing Ke Xue. 2024. PMID: 38216500 Chinese.
-
Bacterial Communities on the Surface of the Mineral Sandy Soil from the Desert of Maine (USA).Curr Microbiol. 2020 Aug;77(8):1429-1437. doi: 10.1007/s00284-020-01946-z. Epub 2020 Mar 19. Curr Microbiol. 2020. PMID: 32193606
-
A systematic review on the morphology structure, propagation characteristics, resistance physiology and exploitation and utilization of Nitraria tangutorum Bobrov.PeerJ. 2024 Aug 16;12:e17830. doi: 10.7717/peerj.17830. eCollection 2024. PeerJ. 2024. PMID: 39161968 Free PMC article.
Cited by
-
Isolation and Identification of Endophytic Bacterial Isolates from the Leaves, Roots, and Stems Parts of Artemisia annua, Moringa oleifera, and Ocimum lamiifolium Plants.Curr Microbiol. 2023 Nov 6;80(12):405. doi: 10.1007/s00284-023-03513-8. Curr Microbiol. 2023. PMID: 37930451
-
Comparative analysis of the microbiomes of strawberry wild species Fragaria nilgerrensis and cultivated variety Akihime using amplicon-based next-generation sequencing.Front Microbiol. 2024 May 30;15:1377782. doi: 10.3389/fmicb.2024.1377782. eCollection 2024. Front Microbiol. 2024. PMID: 38873161 Free PMC article.
-
Microbiome Signature of Endophytes in Wheat Seed Response to Wheat Dwarf Bunt Caused by Tilletia controversa Kühn.Microbiol Spectr. 2023 Feb 14;11(1):e0039022. doi: 10.1128/spectrum.00390-22. Epub 2023 Jan 10. Microbiol Spectr. 2023. PMID: 36625645 Free PMC article.
-
Analysis of the Antioxidant Composition of Low Molecular Weight Metabolites from the Agarolytic Bacterium Alteromonas macleodii QZ9-9: Possibilities for High-Added Value Utilization of Macroalgae.Antioxidants (Basel). 2022 Oct 3;11(10):1977. doi: 10.3390/antiox11101977. Antioxidants (Basel). 2022. PMID: 36290700 Free PMC article.
-
Distinct Bacterial Communities Within the Nonrhizosphere, Rhizosphere, and Endosphere of Ammodendron bifolium Under Winter Condition in the Takeermohuer Desert.Microb Ecol. 2024 Nov 29;87(1):151. doi: 10.1007/s00248-024-02462-4. Microb Ecol. 2024. PMID: 39611982 Free PMC article.
References
-
- Veron S, Paruelo J, Oesterheld M. Assessing desertification. J Arid Environ. 2006;66(4):751–763. doi: 10.1016/j.jaridenv.2006.01.021. - DOI
-
- Huang J, Zhang G, Zhang Y, Guan X, Wei Y, Guo R. Global desertification vulnerability to climate change and human activities. Land Degrad Dev. 2020;31(11):1380–1391. doi: 10.1002/ldr.3556. - DOI
-
- Zhang Y, Cao C, Han X, Jiang S. Soil nutrient and microbiological property recoveries via native shrub and semi-shrub plantations on moving sand dunes in Northeast China. Ecol Eng. 2013;53:1–5. doi: 10.1016/j.ecoleng.2013.01.012. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

