A rodent model of human dose-equivalent progestin-only implantable contraception
- PMID: 33752672
- PMCID: PMC7983206
- DOI: 10.1186/s12958-021-00729-w
A rodent model of human dose-equivalent progestin-only implantable contraception
Abstract
Background: Long-acting, reversible contraceptives (LARC; progestin only) are an increasingly common hormonal contraceptive choice in reproductive aged women looking to suppress ovarian function and menstrual cyclicity. The overall objective was to develop and validate a rodent model of implanted etonogestrel (ENG) LARC, at body size equivalent doses to the average dose received by women during each of the first 3 years of ENG subdermal rod LARC use.
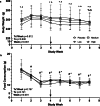
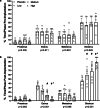
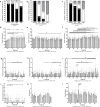
Methods: Intact, virgin, female Sprague-Dawley rats (16-wk-old) were randomized to 1 of 4 groups (n = 8/group) of ENG LARC (high-0.30μg/d, medium-0.17μg/d, low-0.09μg/d, placebo-0.00μg/d) via a slow-release pellet implanted subcutaneously. Animals were monitored for 21 days before and 29 days following pellet implantation using vaginal smears, ultrasound biomicroscopy (UBM), saphenous blood draws, food consumption, and body weights. Data were analyzed by chi-square, non-parametric, univariate, and repeated measures 2-way ANOVA.
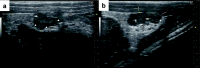
Results: Prior to pellet implantation there was no difference in time spent in estrus cycle phases among the treatment groups (p > 0.30). Following pellet implantation there was a dose-dependent impact on the time spent in diestrus and estrus (p < 0.05), with the high dose group spending more days in diestrus and fewer days in estrus. Prior to pellet insertion there was not an association between treatment group and estrus cycle classification (p = 0.57) but following pellet implantation there was a dose-dependent association with cycle classification (p < 0.02). Measurements from the UBM (ovarian volume, follicle count, corpora lutea count) indicate an alteration of ovarian function following pellet implantation.
Conclusion: Assessment of estrus cyclicity indicated a dose-response relationship in the shift to a larger number of acyclic rats and longer in duration spent in the diestrus phase. Therefore, each dose in this model mimics some of the changes observed in the ovaries of women using ENG LARC and provides an opportunity for investigating the impacts on non-reproductive tissues in the future.
Keywords: Estrus cycle; Etonogestrel; Hormonal contraception; Long-acting reversible contraception; Ovarian function; Progestin; Ultrasound biomicroscopy.
Conflict of interest statement
JI is an employee of Synergyne Imaging Technology, Inc. (Saskatoon, Saskatchewan, Canada). RAP has equity and board positions in Synergyne Imaging Technology, Inc. (Saskatoon, Saskatchewan, Canada). HCMA and SAB declare that they have no competing interests.
Figures
References
-
- Daniels K, Abma JC. NCHS Data Brief, no 327. Hyattsville, MD: National Center for Health Statistics; 2018. Current contraceptive status among women aged 15–49: United States, 2015–2017. NCHS data brief, no 327.
-
- Ali M, Akin A, Bahamondes L, Brache V, Habib N, Landoulsi S, Hubacher D, women WHOsgoscif Extended use up to 5 years of the etonogestrel-releasing subdermal contraceptive implant: comparison to levonorgestrel-releasing subdermal implant. Hum Reprod. 2016;31(11):2491–2498. doi: 10.1093/humrep/dew222. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

