Renaissance of armored immune effector cells, CAR-NK cells, brings the higher hope for successful cancer therapy
- PMID: 33752707
- PMCID: PMC7983395
- DOI: 10.1186/s13287-021-02251-7
Renaissance of armored immune effector cells, CAR-NK cells, brings the higher hope for successful cancer therapy
Abstract
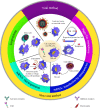
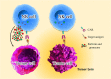
In recent decades, a new method of cellular immunotherapy was introduced based on engineering and empowering the immune effector cells. In this type of immunotherapy, the immune effector cells are equipped with chimeric antigen receptor (CAR) to specifically target cancer cells. In much of the trials and experiments, CAR-modified T cell immunotherapy has achieved very promising therapeutic results in the treatment of some types of cancers and infectious diseases. However, there are also some considerable drawbacks in the clinical application of CAR-T cells although much effort is in progress to rectify the issues. In some conditions, CAR-T cells initiate over-activated and strong immune responses, therefore, causing unexpected side-effects such as systemic cytokine toxicity (i.e., cytokine release syndrome), neurotoxicity, on-target, off-tumor toxicity, and graft-versus-host disease (GvHD). To overcome these limitations in CAR-T cell immunotherapy, NK cells as an alternative source of immune effector cells have been utilized for CAR-engineering. Natural killer cells are key players of the innate immune system that can destroy virus-infected cells, tumor cells, or other aberrant cells with their efficient recognizing capability. Compared to T cells, CAR-transduced NK cells (CAR-NK) have several advantages, such as safety in clinical use, non-MHC-restricted recognition of tumor cells, and renewable and easy cell sources for their preparation. In this review, we will discuss the recent preclinical and clinical studies, different sources of NK cells, transduction methods, possible limitations and challenges, and clinical considerations.
Keywords: CAR; Immunotherapy; NK cells; T cells.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Maoz M, Devir M, Inbar M, Inbar-Daniel Z, Sherill-Rofe D, Bloch I, Meir K, Edelman D, Azzam S, Nechushtan H, Maimon O, Uziely B, Kadouri L, Sonnenblick A, Eden A, Peretz T, Zick A. Clinical implications of sub-grouping HER2 positive tumors by amplicon structure and co-amplified genes. Sci Rep. 2019;9:18795. doi: 10.1038/s41598-019-55455-6. - DOI - PMC - PubMed
-
- Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

