Human placenta mesenchymal stem cell-derived exosomes delay H2O2-induced aging in mouse cholangioids
- PMID: 33752720
- PMCID: PMC7983269
- DOI: 10.1186/s13287-021-02271-3
Human placenta mesenchymal stem cell-derived exosomes delay H2O2-induced aging in mouse cholangioids
Abstract
Background: Cholangiocyte senescence is an important pathological process in diseases such as primary sclerosing cholangitis (PSC) and primary biliary cirrhosis (PBC). Stem cell/induced pluripotent stem cell-derived exosomes have shown anti-senescence effects in various diseases. We applied novel organoid culture technology to establish and characterize cholangiocyte organoids (cholangioids) with oxidative stress-induced senescence and then investigated whether human placenta mesenchymal stem cell (hPMSC)-derived exosomes exerted a protective effect in senescent cholangioids.
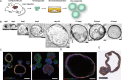
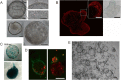
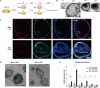
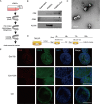
Methods: We identified the growth characteristics of cholangioids by light microscopy and confocal microscopy. Exosomes were introduced concurrently with H2O2 into the cholangioids. Using immunohistochemistry and immunofluorescence staining analyses, we assessed the expression patterns of the senescence markers p16INK4a, p21WAF1/Cip1, and senescence-associated β-galactosidase (SA-β-gal) and then characterized the mRNA and protein expression levels of chemokines and senescence-associated secretory phenotype (SASP) components.
Results: Well-established cholangioids expressed cholangiocyte-specific markers. Oxidative stress-induced senescence enhanced the expression of the senescence-associated proteins p16INK4a, p21WAF1/Cip1, and SA-β-gal in senescent cholangioids compared with the control group. Treatment with hPMSC-derived exosomes delayed the cholangioid aging progress and reduced the levels of SASP components (i.e., interleukin-6 and chemokine CC ligand 2).
Conclusions: Senescent organoids are a potential novel model for better understanding senescence progression in cholangiocytes. hPMSC-derived exosomes exert protective effects against senescent cholangioids under oxidative stress-induced injury by delaying aging and reducing SASP components, which might have therapeutic potential for PSC or PBC.
Keywords: Cholangiocytes; Exosomes; Organoids; Primary sclerosing cholangitis; Senescence.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 2007;8(9):729–740, DOI: 10.1038/nrm2233. - PubMed
-
- Pinto C, Giordano DM, Maroni L, Marzioni M. Role of inflammation and proinflammatory cytokines in cholangiocyte pathophysiology. Biochimica et biophysica acta Molecular basis of disease. 2018;1864(4 Pt B):1270–1278. - PubMed
-
- Chung BK, Karlsen TH, Folseraas T. Cholangiocytes in the pathogenesis of primary sclerosing cholangitis and development of cholangiocarcinoma. Biochimica et biophysica acta Molecular basis of disease. 2018;1864(4 Pt B):1390–1400. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

