Improvement of ECM-based bioroot regeneration via N-acetylcysteine-induced antioxidative effects
- PMID: 33752756
- PMCID: PMC7986250
- DOI: 10.1186/s13287-021-02237-5
Improvement of ECM-based bioroot regeneration via N-acetylcysteine-induced antioxidative effects
Abstract
Background: The low survival rate or dysfunction of extracellular matrix (ECM)-based engineered organs caused by the adverse effects of unfavourable local microenvironments on seed cell viability and stemness, especially the effects of excessive reactive oxygen species (ROS), prompted us to examine the importance of controlling oxidative damage for tissue transplantation and regeneration. We sought to improve the tolerance of seed cells to the transplant microenvironment via antioxidant pathways, thus promoting transplant efficiency and achieving better tissue regeneration.
Methods: We improved the antioxidative properties of ECM-based bioroots with higher glutathione contents in dental follicle stem cells (DFCs) by pretreating cells or loading scaffolds with the antioxidant NAC. Additionally, we developed an in situ rat alveolar fossa implantation model to evaluate the long-term therapeutic effects of NAC in bioroot transplantation.
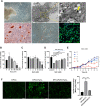
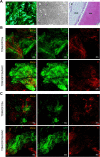
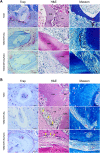
Results: The results showed that NAC decreased H2O2-induced cellular damage and maintained the differentiation potential of DFCs. The transplantation experiments further verified that NAC protected the biological properties of DFCs by repressing replacement resorption or ankylosis, thus facilitating bioroot regeneration.
Conclusions: The following findings suggest that NAC could significantly protect stem cell viability and stemness during oxidative stress and exert better and prolonged effects in bioroot intragrafts.
Keywords: Bioroot regeneration; Dental follicle stem cell; N-Acetylcysteine; Oxidative stress; Treated dentin matrix.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

