Kinetochore assembly throughout the cell cycle
- PMID: 33753005
- PMCID: PMC8384650
- DOI: 10.1016/j.semcdb.2021.03.008
Kinetochore assembly throughout the cell cycle
Abstract
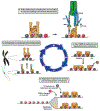
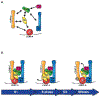
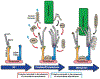
The kinetochore plays an essential role in facilitating chromosome segregation during cell division. This massive protein complex assembles onto the centromere of chromosomes and enables their attachment to spindle microtubules during mitosis. The kinetochore also functions as a signaling hub to regulate cell cycle progression, and is crucial to ensuring the fidelity of chromosome segregation. Despite the fact that kinetochores are large and robust molecular assemblies, they are also highly dynamic structures that undergo structural and organizational changes throughout the cell cycle. This review will highlight our current understanding of kinetochore structure and function, focusing on the dynamic processes that underlie kinetochore assembly.
Keywords: Centromere; Kinetochore; Mitosis.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declarations of interest: none
Figures
References
-
- Maddox PS, Oegema K, Desai A, Cheeseman IM, “Holo”er than thou: Chromosome segregation and kinetochore function in C. elegans, Chromosome Res 12(6) (2004) 641–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

