Altered endocytosis in cellular senescence
- PMID: 33753287
- PMCID: PMC8131247
- DOI: 10.1016/j.arr.2021.101332
Altered endocytosis in cellular senescence
Abstract
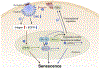
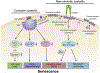
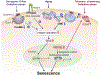
Cellular senescence occurs in response to diverse stresses (e.g., telomere shortening, DNA damage, oxidative stress, oncogene activation). A growing body of evidence indicates that alterations in multiple components of endocytic pathways contribute to cellular senescence. Clathrin-mediated endocytosis (CME) and caveolae-mediated endocytosis (CavME) represent major types of endocytosis that are implicated in senescence. More recent research has also identified a chromatin modifier and tumor suppressor that contributes to the induction of senescence via altered endocytosis. Here, molecular regulators of aberrant endocytosis-induced senescence are reviewed and discussed in the context of their capacity to serve as senescence-inducing stressors or modifiers.
Keywords: Amphiphysin; Caveolin-1; Endocytosis; ING1; Senescence; βPAK-interacting nucleotide exchange factor (βPIX).
Copyright © 2021 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors report no declarations of interest.
Figures

References
-
- Abad M, Menendez C, Fuchtbauer A, Serrano M, Fuchtbauer EM, Palmero I, 2007. Ing1 mediates p53 accumulation and chromatin modification in response to oncogenic stress. J. Biol. Chem 282, 31060–31067. - PubMed
-
- Abad M, Moreno A, Palacios A, Narita M, Blanco F, Moreno-Bueno G, Narita M, Palmero I, 2011. The tumor suppressor ING1 contributes to epigenetic control of cellular senescence. Aging Cell 10, 158–171. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

