Assessing the Bioactive Profile of Antifungal-Loaded Calcium Sulfate against Fungal Biofilms
- PMID: 33753336
- PMCID: PMC8316021
- DOI: 10.1128/AAC.02551-20
Assessing the Bioactive Profile of Antifungal-Loaded Calcium Sulfate against Fungal Biofilms
Abstract
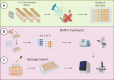
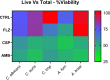
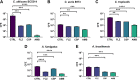
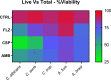
Calcium sulfate (CS) has been used clinically as a bone- or void-filling biomaterial, and its resorptive properties have provided the prospect for its use as a release mechanism for local antibiotics to control biofilms. Here, we aimed to test CS beads loaded with three antifungal drugs against planktonic and sessile fungal species to assess whether these antifungal beads could be harnessed to provide consistent release of antifungals at biofilm-inhibitory doses. A panel of different fungal species (n = 15) were selected for planktonic broth microdilution testing with fluconazole (FLZ), amphotericin B (AMB), and caspofungin (CSP). After establishing planktonic inhibition, antifungal CS beads were introduced to fungal biofilms (n = 5) to assess biofilm formation and cell viability through a combination of standard quantitative and qualitative biofilm assays. Inoculation of a hydrogel substrate, packed with antifungal CS beads, was also used to assess diffusion through a semidry material, to mimic active infection in vivo In general, antifungals released from loaded CS beads were all effective at inhibiting the pathogenic fungi over 7 days within standard MIC ranges for these fungi. We observed a significant reduction of pregrown fungal biofilms across key fungal pathogens following treatment, with visually observable changes in cell morphology and biofilm coverage provided by scanning electron microscopy. Assessment of biofilm inhibition also revealed reductions in total and viable cells across all organisms tested. These data show that antifungal-loaded CS beads produce a sustained antimicrobial effect that inhibits and kills clinically relevant fungal species in vitro as planktonic and biofilm cells.
Keywords: Candida; antimicrobial agents; biofilm; fungal; joint infections; wound management.
Copyright © 2021 Butcher et al.
Figures




References
-
- Ranawat CS, Flynn WF, Jr, Saddler S, Hansraj KK, Maynard MJ. 1993. Long-term results of the total condylar knee arthroplasty. A 15-year survivorship study. Clin Orthop Relat Res 286:94–102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

