GLUT1 Expression in Tumor-Associated Neutrophils Promotes Lung Cancer Growth and Resistance to Radiotherapy
- PMID: 33753374
- PMCID: PMC8137580
- DOI: 10.1158/0008-5472.CAN-20-2870
GLUT1 Expression in Tumor-Associated Neutrophils Promotes Lung Cancer Growth and Resistance to Radiotherapy
Abstract
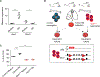
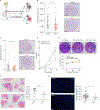
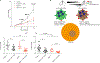
Neutrophils are the most abundant circulating leucocytes and are essential for innate immunity. In cancer, pro- or antitumor properties have been attributed to tumor-associated neutrophils (TAN). Here, focusing on TAN accumulation within lung tumors, we identify GLUT1 as an essential glucose transporter for their tumor supportive behavior. Compared with normal neutrophils, GLUT1 and glucose metabolism increased in TANs from a mouse model of lung adenocarcinoma. To elucidate the impact of glucose uptake on TANs, we used a strategy with two recombinases, dissociating tumor initiation from neutrophil-specific Glut1 deletion. Loss of GLUT1 accelerated neutrophil turnover in tumors and reduced a subset of TANs expressing SiglecF. In the absence of GLUT1 expression by TANs, tumor growth was diminished and the efficacy of radiotherapy was augmented. Our results demonstrate the importance of GLUT1 in TANs, which may affect their pro- versus antitumor behavior. These results also suggest targeting metabolic vulnerabilities to favor antitumor neutrophils. SIGNIFICANCE: Lung tumor support and radiotherapy resistance depend on GLUT1-mediated glucose uptake in tumor-associated neutrophils, indicating that metabolic vulnerabilities should be considered to target both tumor cells as well as innate immune cells. GRAPHICAL ABSTRACT: http://cancerres.aacrjournals.org/content/canres/81/9/2345/F1.large.jpg.
©2021 American Association for Cancer Research.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

