Lymphotoxin β Receptor: a Crucial Role in Innate and Adaptive Immune Responses against Toxoplasma gondii
- PMID: 33753412
- PMCID: PMC8316152
- DOI: 10.1128/IAI.00026-21
Lymphotoxin β Receptor: a Crucial Role in Innate and Adaptive Immune Responses against Toxoplasma gondii
Abstract
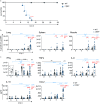
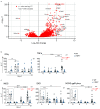
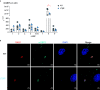
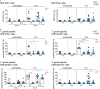
The lymphotoxin β receptor (LTβR) plays an essential role in the initiation of immune responses to intracellular pathogens. In mice, the LTβR is crucial for surviving acute toxoplasmosis; however, until now, a functional analysis was largely incomplete. Here, we demonstrate that the LTβR is a key regulator required for the intricate balance of adaptive immune responses. Toxoplasma gondii-infected LTβR-deficient (LTβR-/-) mice show globally altered interferon-γ (IFN-γ) regulation, reduced IFN-γ-controlled host effector molecule expression, impaired T cell functionality, and an absent anti-parasite-specific IgG response, resulting in a severe loss of immune control of the parasites. Reconstitution of LTβR-/- mice with toxoplasma immune serum significantly prolongs survival following T. gondii infection. Notably, analysis of RNA-seq data clearly indicates a specific effect of T. gondii infection on the B cell response and isotype switching. This study uncovers the decisive role of the LTβR in cytokine regulation and adaptive immune responses to control T. gondii.
Keywords: Toxoplasma gondii; host-pathogen interactions; lymphotoxin.
Copyright © 2021 Tersteegen et al.
Figures



Similar articles
-
The Lymphotoxin β Receptor Is Essential for Upregulation of IFN-Induced Guanylate-Binding Proteins and Survival after Toxoplasma gondii Infection.Mediators Inflamm. 2017;2017:7375818. doi: 10.1155/2017/7375818. Epub 2017 Aug 6. Mediators Inflamm. 2017. PMID: 28845089 Free PMC article.
-
Essential Role of mGBP7 for Survival of Toxoplasma gondii Infection.mBio. 2020 Jan 21;11(1):e02993-19. doi: 10.1128/mBio.02993-19. mBio. 2020. PMID: 31964735 Free PMC article.
-
Epigenetic Control of IFN-γ Host Responses During Infection With Toxoplasma gondii.Front Immunol. 2020 Sep 25;11:581241. doi: 10.3389/fimmu.2020.581241. eCollection 2020. Front Immunol. 2020. PMID: 33072127 Free PMC article.
-
Innate responses to Toxoplasma gondii in mice and humans.Trends Parasitol. 2011 Sep;27(9):388-93. doi: 10.1016/j.pt.2011.03.009. Trends Parasitol. 2011. PMID: 21550851 Free PMC article. Review.
-
Immune response and immunopathology during toxoplasmosis.Semin Immunopathol. 2012 Nov;34(6):793-813. doi: 10.1007/s00281-012-0339-3. Epub 2012 Sep 7. Semin Immunopathol. 2012. PMID: 22955326 Free PMC article. Review.
Cited by
-
The emerging role of Toxoplasma gondii in periodontal diseases and underlying mechanisms.Front Immunol. 2024 Oct 3;15:1464108. doi: 10.3389/fimmu.2024.1464108. eCollection 2024. Front Immunol. 2024. PMID: 39430742 Free PMC article. Review.
-
The Regulation of Neutrophil Migration in Patients with Sepsis: The Complexity of the Molecular Mechanisms and Their Modulation in Sepsis and the Heterogeneity of Sepsis Patients.Cells. 2023 Mar 24;12(7):1003. doi: 10.3390/cells12071003. Cells. 2023. PMID: 37048076 Free PMC article. Review.
References
-
- Ehlers S, Holscher C, Scheu S, Tertilt C, Hehlgans T, Suwinski J, Endres R, Pfeffer K. 2003. The lymphotoxin beta receptor is critically involved in controlling infections with the intracellular pathogens Mycobacterium tuberculosis and Listeria monocytogenes. J Immunol 170:5210–5218. 10.4049/jimmunol.170.10.5210. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

