Seroepidemiology of hepatitis A and B in the general population in Hong Kong: protocol of a cross-sectional survey using spatial sampling in a highly urbanised city
- PMID: 33753433
- PMCID: PMC7986661
- DOI: 10.1136/bmjopen-2020-042065
Seroepidemiology of hepatitis A and B in the general population in Hong Kong: protocol of a cross-sectional survey using spatial sampling in a highly urbanised city
Abstract
Introduction: Differences in immunisation policies have significantly reshaped the epidemiology of hepatitis A and B in the population. Assessment of the susceptibility and transmission potential of these two types of vaccine-preventable hepatitis would enhance the capacity of public health authorities for viral hepatitis elimination. Focusing on Hong Kong, the objectives of this study comprise the determination of the population-level seroprevalence of hepatitis A and B and an examination of the risk factors for virus transmission and the population impacts of vaccinations.
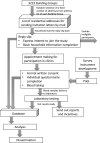
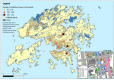
Methods and analysis: This is a cross-sectional household survey on hepatitis A and B. By using socially homogeneous building groups as sampling frame, eligible members of 1327 spatially selected households would be invited to complete a questionnaire and provide blood samples for serological testing (anti-hepatitis A virus, hepatitis B surface antigen, hepatitis B surface and core antibody). The main measures comprise a set of metrics on the prevalence of hepatitis A and B. Analysis would be conducted to examine the association of risk factors with the tested markers and describe the attitudes towards viral hepatitis vaccination.
Ethics and dissemination: Ethical approval from the Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee, and approval for laboratory safety from the Chinese University of Hong Kong have been obtained. The study results will be presented in scientific forums to update on the epidemiology of hepatitis A and B and inform the development of new vaccination strategies in Hong Kong.
Trial registration number: NCT04371276.
Keywords: epidemiology; hepatology; immunology; public health.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- World Health Organization . Global hepatitis report, 2017. Available: https://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-en... [Accessed 4 May 2020].
-
- World Health Organization . Regional office for the Western Pacific. Regional action plan for viral hepatitis in the Western Pacific 2016-2020: a priority action plan for awareness, surveillance, prevention and treatment of viral hepatitis in the Western Pacific Region. Available: https://apps.who.int/iris/bitstream/handle/10665/208337/97892906177617_e... [Accessed 4 May 2020].
-
- Centre for Health Protection, Department of Health . Surveillance of viral hepatitis in Hong Kong – 2018 report. Available: https://www.chp.gov.hk/files/pdf/viral_hep_sur_report_2018.pdf [Accessed on 22 Apr 2020].
-
- Poon C. Update on hepatitis A in Hong Kong. Communicable Diseases Watch 2015;12:65–7.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical