Neoadjuvant Pembrolizumab and High-Dose IFNα-2b in Resectable Regionally Advanced Melanoma
- PMID: 33753453
- PMCID: PMC8338751
- DOI: 10.1158/1078-0432.CCR-20-4301
Neoadjuvant Pembrolizumab and High-Dose IFNα-2b in Resectable Regionally Advanced Melanoma
Abstract
Purpose: Neoadjuvant immunotherapy may improve the clinical outcome of regionally advanced operable melanoma and allows for rapid clinical and pathologic assessment of response. We examined neoadjuvant pembrolizumab and high-dose IFNα-2b (HDI) therapy in patients with resectable advanced melanoma.
Patients and methods: Patients with resectable stage III/IV melanoma were treated with concurrent pembrolizumab 200 mg i.v. every 3 weeks and HDI 20 MU/m2/day i.v., 5 days per week for 4 weeks, then 10 MU/m2/day subcutaneously 3 days per week for 2 weeks. Definitive surgery followed, as did adjuvant combination immunotherapy, completing a year of treatment. Primary endpoint was safety of the combination. Secondary endpoints included overall response rate (ORR), pathologic complete response (pCR), recurrence-free survival (RFS), and overall survival (OS). Blood samples for correlative studies were collected throughout. Tumor tissue was assessed by IHC and flow cytometry at baseline and at surgery.
Results: A total of 31 patients were enrolled, and 30 were evaluable. At data cutoff (October 2, 2019), median follow-up for OS was 37.87 months (range, 33.2-43.47). Median OS and RFS were not reached. Radiographic ORR was 73.3% [95% confidence interval (CI): 55.5-85.8], with a 43% (95% CI: 27.3-60.1) pCR rate. None of the patients with a pCR have had a recurrence. HDI and pembrolizumab were discontinued in 73% and 43% of patients, respectively. Correlative analyses suggested that intratumoral PD-1/PD-L1 interaction and HLA-DR expression are associated with pCR (P = 0.002 and P = 0.008, respectively).
Conclusions: Neoadjuvant concurrent HDI and pembrolizumab demonstrated promising clinical activity despite high rates of treatment discontinuation. pCR is a prognostic indicator.See related commentary by Menzies et al., p. 4133.
©2021 American Association for Cancer Research.
Conflict of interest statement
The remaining authors declare no conflicts of interest.
Figures

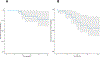
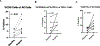
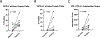
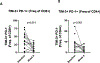

Comment in
-
Neoadjuvant Immunotherapy in Melanoma - The New Frontier.Clin Cancer Res. 2021 Aug 1;27(15):4133-4135. doi: 10.1158/1078-0432.CCR-21-1236. Epub 2021 Jun 3. Clin Cancer Res. 2021. PMID: 34083235
References
-
- National Comprehensive Cancer Network: Cutaneous Melanoma (Version 1.2020), 2020
-
- Long GV, Hauschild A, Santinami M, et al.: Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N Engl J Med 377:1813–1823, 2017 - PubMed
-
- Weber J, Glutsch V, Geissinger E, et al.: Neoadjuvant immunotherapy with combined ipilimumab and nivolumab in patients with melanoma with primary or in transit disease. Br J Dermatol, 2019 - PubMed
-
- Rozeman EA, Menzies AM, van Akkooi ACJ, et al.: Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol 20:948–960, 2019 - PubMed
-
- Menzies AM, Rozeman EA, Amaria RN, et al.: Pathological response and survival with neoadjuvant therapy in melanoma: A pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Journal of Clinical Oncology 37:9503–9503, 2019 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

