Adjuvant Sirolimus Does Not Improve Outcome in Pet Dogs Receiving Standard-of-Care Therapy for Appendicular Osteosarcoma: A Prospective, Randomized Trial of 324 Dogs
- PMID: 33753454
- PMCID: PMC8172450
- DOI: 10.1158/1078-0432.CCR-21-0315
Adjuvant Sirolimus Does Not Improve Outcome in Pet Dogs Receiving Standard-of-Care Therapy for Appendicular Osteosarcoma: A Prospective, Randomized Trial of 324 Dogs
Abstract
Purpose: The mTOR pathway has been identified as a key nutrient signaling hub that participates in metastatic progression of high-grade osteosarcoma. Inhibition of mTOR signaling is biologically achievable with sirolimus, and might slow the outgrowth of distant metastases. In this study, pet dogs with appendicular osteosarcoma were leveraged as high-value biologic models for pediatric osteosarcoma, to assess mTOR inhibition as a therapeutic strategy for attenuating metastatic disease progression.
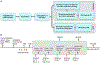
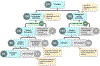
Patients and methods: A total of 324 pet dogs diagnosed with treatment-naïve appendicular osteosarcoma were randomized into a two-arm, multicenter, parallel superiority trial whereby dogs received amputation of the affected limb, followed by adjuvant carboplatin chemotherapy ± oral sirolimus therapy. The primary outcome measure was disease-free interval (DFI), as assessed by serial physical and radiologic detection of emergent macroscopic metastases; secondary outcomes included overall 1- and 2-year survival rates, and sirolimus pharmacokinetic variables and their correlative relationship to adverse events and clinical outcomes.
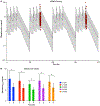
Results: There was no significant difference in the median DFI or overall survival between the two arms of this trial; the median DFI and survival for standard-of-care (SOC; defined as amputation and carboplatin therapy) dogs was 180 days [95% confidence interval (CI), 144-237] and 282 days (95% CI, 224-383) and for SOC + sirolimus dogs, it was 204 days (95% CI, 157-217) and 280 days (95% CI, 252-332), respectively.
Conclusions: In a population of pet dogs nongenomically segmented for predicted mTOR inhibition response, sequentially administered adjuvant sirolimus, although well tolerated when added to a backbone of therapy, did not extend DFI or survival in dogs with appendicular osteosarcoma.
©2021 American Association for Cancer Research.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
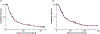
References
-
- Angstadt AY, Thayanithy V, Subramanian S, Modiano JF, Breen M. A genome-wide approach to comparative oncology: high-resolution oligonucleotide aCGH of canine and human osteosarcoma pinpoints shared microaberrations. Cancer Genet 2012;205(11):572–87 doi 10.1016/j.cancergen.2012.09.005. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

