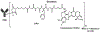FDA Approval Summary: Fam-Trastuzumab Deruxtecan-Nxki for the Treatment of Unresectable or Metastatic HER2-Positive Breast Cancer
- PMID: 33753456
- PMCID: PMC8570919
- DOI: 10.1158/1078-0432.CCR-20-4557
FDA Approval Summary: Fam-Trastuzumab Deruxtecan-Nxki for the Treatment of Unresectable or Metastatic HER2-Positive Breast Cancer
Abstract
On December 20, 2019, the FDA granted accelerated approval to fam-trastuzumab deruxtecan-nxki [DS-8201a; T-DXd; tradename ENHERTU (Daiichi Sankyo)] for the treatment of adult patients with unresectable or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2-based regimens in the metastatic setting. Approval was based on data from study DS8201-A-U201 (DESTINY-Breast01) with supportive safety data from study DS8201-A-J101. The primary efficacy endpoint in DESTINY-Breast01 was overall response rate (ORR) based on confirmed responses by blinded independent central review (ICR) using RECIST v1.1 in all participants who were assigned to receive the recommended dose of 5.4 mg/kg while secondary endpoints included duration of response (DoR). The confirmed ORR based on ICR in these 184 patients was 60.3% [95% confidence interval (CI): 52.9-67.4] and the median DoR was 14.8 months (95% CI: 13.8-16.9). Interstitial lung disease, including pneumonitis, was experienced in patients treated with T-DXd and can be severe, life threatening, or fatal. In addition, neutropenia and left ventricular dysfunction were included as Warnings and Precautions in labeling. Other important common adverse reactions were nausea, fatigue, vomiting, alopecia, constipation, decreased appetite, anemia, diarrhea, and thrombocytopenia. Overall, the totality of efficacy and safety data supported the accelerated approval of T-DXd for the intended indication.
©2021 American Association for Cancer Research.
Conflict of interest statement
Figures
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68(6):394–424. - PubMed
-
- National Cancer Institute. Surveillance, Epidemiology and End Results Program (SEER). Female Breast Cancer. [cited 2020 Jul 2]. Available from: https://seer.cancer.gov/statfacts/html/breast.html
-
- American Cancer Society. Key Statistics for Breast Cancer in Men. [cited 2020 Jul 2]. Available from: https://www.cancer.org/cancer/breast-cancer-in-men/about/key-statistics....
-
- National Cancer Institute. Surveillance, Epidemiology and End Results Program (SEER). Female Breast Cancer Subtypes. [cited 2020 Jun 2]. Available from: https://seer.cancer.gov/statfacts/html/breast-subtypes.html
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous