Paxbp1 controls a key checkpoint for cell growth and survival during early activation of quiescent muscle satellite cells
- PMID: 33753492
- PMCID: PMC8020634
- DOI: 10.1073/pnas.2021093118
Paxbp1 controls a key checkpoint for cell growth and survival during early activation of quiescent muscle satellite cells
Abstract
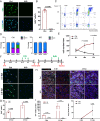
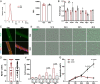
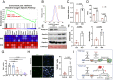
Adult mouse muscle satellite cells (MuSCs) are quiescent in uninjured muscles. Upon muscle injury, MuSCs exit quiescence, reenter the cell cycle to proliferate and self-renew, and then differentiate and fuse to drive muscle regeneration. However, it remains poorly understood how MuSCs transition from quiescence to the cycling state. Here, we report that Pax3 and Pax7 binding protein 1 (Paxbp1) controls a key checkpoint during this critical transition. Deletion of Paxbp1 in adult MuSCs prevented them from reentering the cell cycle upon injury, resulting in a total regeneration failure. Mechanistically, we found an abnormal elevation of reactive oxygen species (ROS) in Paxbp1-null MuSCs, which induced p53 activation and impaired mTORC1 signaling, leading to defective cell growth, apoptosis, and failure in S-phase reentry. Deliberate ROS reduction partially rescued the cell-cycle reentry defect in mutant MuSCs. Our study reveals that Paxbp1 regulates a late cell-growth checkpoint essential for quiescent MuSCs to reenter the cell cycle upon activation.
Keywords: Paxbp1; ROS; cell growth; muscle satellite cells; quiescence.
Conflict of interest statement
The authors declare no competing interest.
Figures



Similar articles
-
A Long Journey before Cycling: Regulation of Quiescence Exit in Adult Muscle Satellite Cells.Int J Mol Sci. 2022 Feb 3;23(3):1748. doi: 10.3390/ijms23031748. Int J Mol Sci. 2022. PMID: 35163665 Free PMC article. Review.
-
p110α of PI3K is necessary and sufficient for quiescence exit in adult muscle satellite cells.EMBO J. 2018 Apr 13;37(8):e98239. doi: 10.15252/embj.201798239. Epub 2018 Mar 26. EMBO J. 2018. PMID: 29581096 Free PMC article.
-
PAX3 Confers Functional Heterogeneity in Skeletal Muscle Stem Cell Responses to Environmental Stress.Cell Stem Cell. 2019 Jun 6;24(6):958-973.e9. doi: 10.1016/j.stem.2019.03.019. Epub 2019 Apr 18. Cell Stem Cell. 2019. PMID: 31006622 Free PMC article.
-
Dlk1 regulates quiescence in calcitonin receptor-mutant muscle stem cells.Stem Cells. 2021 Mar;39(3):306-317. doi: 10.1002/stem.3312. Epub 2020 Dec 8. Stem Cells. 2021. PMID: 33295098
-
Dormancy and quiescence of skeletal muscle stem cells.Results Probl Cell Differ. 2015;56:215-35. doi: 10.1007/978-3-662-44608-9_10. Results Probl Cell Differ. 2015. PMID: 25344673 Review.
Cited by
-
Implicating effector genes at COVID-19 GWAS loci using promoter-focused Capture-C in disease-relevant immune cell types.Genome Biol. 2022 Jun 3;23(1):125. doi: 10.1186/s13059-022-02691-1. Genome Biol. 2022. PMID: 35659055 Free PMC article.
-
Paxbp1 is Indispensable for the Maintenance of Hair Follicle Homeostasis.Acta Derm Venereol. 2025 May 19;105:adv43648. doi: 10.2340/actadv.v105.43648. Acta Derm Venereol. 2025. PMID: 40390261 Free PMC article. No abstract available.
-
Control of satellite cell function in muscle regeneration and its disruption in ageing.Nat Rev Mol Cell Biol. 2022 Mar;23(3):204-226. doi: 10.1038/s41580-021-00421-2. Epub 2021 Oct 18. Nat Rev Mol Cell Biol. 2022. PMID: 34663964 Review.
-
A Long Journey before Cycling: Regulation of Quiescence Exit in Adult Muscle Satellite Cells.Int J Mol Sci. 2022 Feb 3;23(3):1748. doi: 10.3390/ijms23031748. Int J Mol Sci. 2022. PMID: 35163665 Free PMC article. Review.
-
Intravital microscopy of satellite cell dynamics and their interaction with myeloid cells during skeletal muscle regeneration.Sci Adv. 2023 Oct 20;9(42):eadi1891. doi: 10.1126/sciadv.adi1891. Epub 2023 Oct 18. Sci Adv. 2023. PMID: 37851799 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

