The number of catalytic cycles in an enzyme's lifetime and why it matters to metabolic engineering
- PMID: 33753504
- PMCID: PMC8020674
- DOI: 10.1073/pnas.2023348118
The number of catalytic cycles in an enzyme's lifetime and why it matters to metabolic engineering
Abstract
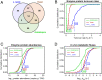
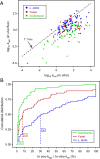
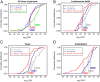
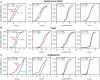
Metabolic engineering uses enzymes as parts to build biosystems for specified tasks. Although a part's working life and failure modes are key engineering performance indicators, this is not yet so in metabolic engineering because it is not known how long enzymes remain functional in vivo or whether cumulative deterioration (wear-out), sudden random failure, or other causes drive replacement. Consequently, enzymes cannot be engineered to extend life and cut the high energy costs of replacement. Guided by catalyst engineering, we adopted catalytic cycles until replacement (CCR) as a metric for enzyme functional life span in vivo. CCR is the number of catalytic cycles that an enzyme mediates in vivo before failure or replacement, i.e., metabolic flux rate/protein turnover rate. We used estimated fluxes and measured protein turnover rates to calculate CCRs for ∼100-200 enzymes each from Lactococcus lactis, yeast, and Arabidopsis CCRs in these organisms had similar ranges (<103 to >107) but different median values (3-4 × 104 in L. lactis and yeast versus 4 × 105 in Arabidopsis). In all organisms, enzymes whose substrates, products, or mechanisms can attack reactive amino acid residues had significantly lower median CCR values than other enzymes. Taken with literature on mechanism-based inactivation, the latter finding supports the proposal that 1) random active-site damage by reaction chemistry is an important cause of enzyme failure, and 2) reactive noncatalytic residues in the active-site region are likely contributors to damage susceptibility. Enzyme engineering to raise CCRs and lower replacement costs may thus be both beneficial and feasible.
Keywords: catalytic cycles; energetic costs; enzyme longevity; protein turnover; synthetic biology.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
Enzymes as Parts in Need of Replacement - and How to Extend Their Working Life.Trends Plant Sci. 2020 Jul;25(7):661-669. doi: 10.1016/j.tplants.2020.02.006. Epub 2020 Mar 23. Trends Plant Sci. 2020. PMID: 32526171 Review.
-
Harnessing the optimization of enzyme catalytic rates in engineering of metabolic phenotypes.PLoS Comput Biol. 2024 Nov 4;20(11):e1012576. doi: 10.1371/journal.pcbi.1012576. eCollection 2024 Nov. PLoS Comput Biol. 2024. PMID: 39495797 Free PMC article.
-
Isobutanol production in engineered Saccharomyces cerevisiae by overexpression of 2-ketoisovalerate decarboxylase and valine biosynthetic enzymes.Bioprocess Biosyst Eng. 2012 Nov;35(9):1467-75. doi: 10.1007/s00449-012-0736-y. Epub 2012 Apr 28. Bioprocess Biosyst Eng. 2012. PMID: 22543927
-
Directed Evolution of a Designer Enzyme Featuring an Unnatural Catalytic Amino Acid.Angew Chem Int Ed Engl. 2019 Feb 11;58(7):2083-2087. doi: 10.1002/anie.201813499. Epub 2019 Jan 14. Angew Chem Int Ed Engl. 2019. PMID: 30575260 Free PMC article.
-
Metabolic engineering and synthetic biology employing Lactococcus lactis and Bacillus subtilis cell factories.Curr Opin Biotechnol. 2019 Oct;59:1-7. doi: 10.1016/j.copbio.2019.01.007. Epub 2019 Feb 18. Curr Opin Biotechnol. 2019. PMID: 30784872 Review.
Cited by
-
Dynamics of Rubisco regulation by sugar phosphate derivatives and their phosphatases.J Exp Bot. 2023 Jan 11;74(2):581-590. doi: 10.1093/jxb/erac386. J Exp Bot. 2023. PMID: 36173669 Free PMC article.
-
In vitro turnover numbers do not reflect in vivo activities of yeast enzymes.Proc Natl Acad Sci U S A. 2021 Aug 10;118(32):e2108391118. doi: 10.1073/pnas.2108391118. Proc Natl Acad Sci U S A. 2021. PMID: 34341111 Free PMC article.
-
Respiratory energy demands and scope for demand expansion and destruction.Plant Physiol. 2023 Apr 3;191(4):2093-2103. doi: 10.1093/plphys/kiac493. Plant Physiol. 2023. PMID: 36271857 Free PMC article.
-
A multi-organ maize metabolic model connects temperature stress with energy production and reducing power generation.iScience. 2023 Nov 7;26(12):108400. doi: 10.1016/j.isci.2023.108400. eCollection 2023 Dec 15. iScience. 2023. PMID: 38077131 Free PMC article.
-
Escherichia coli aceE variants coding pyruvate dehydrogenase improve the generation of pyruvate-derived acetoin.Eng Life Sci. 2023 Jan 31;23(3):e2200054. doi: 10.1002/elsc.202200054. eCollection 2023 Mar. Eng Life Sci. 2023. PMID: 36874610 Free PMC article.
References
-
- Way J. C., Collins J. J., Keasling J. D., Silver P. A., Integrating biological redesign: Where synthetic biology came from and where it needs to go. Cell 157, 151–161 (2014). - PubMed
-
- Silver P. A., Way J. C., Arnold F. H., Meyerowitz J. T., Synthetic biology: Engineering explored. Nature 509, 166–167 (2014). - PubMed
-
- Amadi-Echendu J. E., et al. ., “What is engineering asset management?” in Definitions, Concepts and Scope of Engineering Asset Management, Amadi-Echendu J. E., Brown K., Willett R., Mathew J., Eds. (Springer, 2010), pp. 3–16.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

