Isolation and characterization of Helicobacter suis from human stomach
- PMID: 33753513
- PMCID: PMC8020762
- DOI: 10.1073/pnas.2026337118
Isolation and characterization of Helicobacter suis from human stomach
Abstract
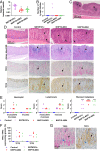
Helicobacter suis, a bacterial species naturally hosted by pigs, can colonize the human stomach in the context of gastric diseases such as gastric mucosa-associated lymphoid tissue (MALT) lymphoma. Because H. suis has been successfully isolated from pigs, but not from humans, evidence linking human H. suis infection to gastric diseases has remained incomplete. In this study, we successfully in vitro cultured H. suis directly from human stomachs. Unlike Helicobacter pylori, the viability of H. suis decreases significantly on neutral pH; therefore, we achieved this using a low-pH medium for transport of gastric biopsies. Ultimately, we isolated H. suis from three patients with gastric diseases, including gastric MALT lymphoma. Successful eradication of H. suis yielded significant improvements in endoscopic and histopathological findings. Oral infection of mice with H. suis clinical isolates elicited gastric and systemic inflammatory responses; in addition, progression of gastric mucosal metaplasia was observed 4 mo postinfection. Because H. suis could be isolated from the stomachs of infected mice, our findings satisfied Koch's postulates. Although further prospective clinical studies are needed, H. suis, like H. pylori, is likely a gastric pathogen in humans. Furthermore, comparative genomic analysis of H. suis using complete genomes of clinical isolates revealed that the genome of each H. suis isolate contained highly plastic genomic regions encoding putative strain-specific virulence factors, including type IV secretion system-associated genes, and that H. suis isolates from humans and pigs were genetically very similar, suggesting possible pig-to-human transmission.
Keywords: Helicobacter suis; gastric diseases; zoonosis.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
Gastric Helicobacter species associated with dogs, cats and pigs: significance for public and animal health.Vet Res. 2022 Jun 13;53(1):42. doi: 10.1186/s13567-022-01059-4. Vet Res. 2022. PMID: 35692057 Free PMC article. Review.
-
Genome sequence of Helicobacter suis supports its role in gastric pathology.Vet Res. 2011 Mar 17;42(1):51. doi: 10.1186/1297-9716-42-51. Vet Res. 2011. PMID: 21414191 Free PMC article.
-
Comparative virulence of in vitro-cultured primate- and pig-associated Helicobacter suis strains in a BALB/c mouse and a Mongolian gerbil model.Helicobacter. 2017 Apr;22(2). doi: 10.1111/hel.12349. Epub 2016 Aug 24. Helicobacter. 2017. PMID: 27558281
-
Helicobacter suis causes severe gastric pathology in mouse and mongolian gerbil models of human gastric disease.PLoS One. 2010 Nov 22;5(11):e14083. doi: 10.1371/journal.pone.0014083. PLoS One. 2010. PMID: 21124878 Free PMC article.
-
Helicobacter heilmannii sensu lato: an overview of the infection in humans.World J Gastroenterol. 2014 Dec 21;20(47):17779-87. doi: 10.3748/wjg.v20.i47.17779. World J Gastroenterol. 2014. PMID: 25548476 Free PMC article. Review.
Cited by
-
Meta-analysis reveals Helicobacter pylori mutual exclusivity and reproducible gastric microbiome alterations during gastric carcinoma progression.Gut Microbes. 2023 Jan-Dec;15(1):2197835. doi: 10.1080/19490976.2023.2197835. Gut Microbes. 2023. PMID: 37020297 Free PMC article.
-
Prospective Multicenter Surveillance of Non-H. pylori Helicobacter Infections during Medical Checkups, Japan.Emerg Infect Dis. 2025 Jun;31(6):1121-1130. doi: 10.3201/eid3106.241315. Emerg Infect Dis. 2025. PMID: 40439414 Free PMC article.
-
Gastric Helicobacter species associated with dogs, cats and pigs: significance for public and animal health.Vet Res. 2022 Jun 13;53(1):42. doi: 10.1186/s13567-022-01059-4. Vet Res. 2022. PMID: 35692057 Free PMC article. Review.
-
Triple-drug combination therapy versus six-month proton pump inhibitor monotherapy in non-Helicobacter pylori Helicobacter eradication, and hyperacid environment preference of Helicobacter suis: a clinical study.BMC Gastroenterol. 2024 May 8;24(1):157. doi: 10.1186/s12876-024-03252-5. BMC Gastroenterol. 2024. PMID: 38720287 Free PMC article.
-
Non-Helicobacter pylori Helicobacter (NHPH) positive gastric cancer.Sci Rep. 2022 Mar 21;12(1):4811. doi: 10.1038/s41598-022-08962-y. Sci Rep. 2022. PMID: 35314746 Free PMC article.
References
-
- Dent J. C., McNulty C. A., Uff J. C., Wilkinson S. P., Gear M. W., Spiral organisms in the gastric antrum. Lancet 2, 96 (1987). - PubMed
-
- Debongnie J. C., et al. ., Gastric ulcers and Helicobacter heilmannii. Eur. J. Gastroenterol. Hepatol. 10, 251–254 (1998). - PubMed
-
- Morgner A., Bayerdörffer E., Meining A., Stolte M., Kroher G., Helicobacter heilmannii and gastric cancer. Lancet 346, 511–512 (1995). - PubMed
-
- Morgner A., et al. ., Helicobacter heilmannii-associated primary gastric low-grade MALT lymphoma: Complete remission after curing the infection. Gastroenterology 118, 821–828 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

