Restriction of food intake by PPP1R17-expressing neurons in the DMH
- PMID: 33753517
- PMCID: PMC8020659
- DOI: 10.1073/pnas.2100194118
Restriction of food intake by PPP1R17-expressing neurons in the DMH
Abstract
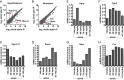
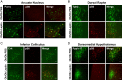
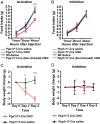
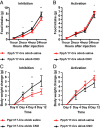
Leptin-deficient ob/ob mice eat voraciously, and their food intake is markedly reduced by leptin treatment. In order to identify potentially novel sites of leptin action, we used PhosphoTRAP to molecularly profile leptin-responsive neurons in the hypothalamus and brainstem. In addition to identifying several known leptin responsive populations, we found that neurons in the dorsomedial hypothalamus (DMH) of ob/ob mice expressing protein phosphatase 1 regulatory subunit 17 (PPP1R17) constitutively express cFos and that this is suppressed by leptin treatment. Because ob mice are hyperphagic, we hypothesized that activating PPP1R17 neurons would increase food intake. However, chemogenetic activation of PPP1R17 neurons decreased food intake and body weight of ob/ob mice while inhibition of PPP1R17 neurons increased them. Similarly, in a scheduled feeding protocol that elicits increased consumption, mice also ate more when PPP1R17 neurons were inhibited and ate less when they were activated. Finally, we found that pair-feeding of ob mice reduced cFos expression to a similar extent as leptin and that reducing the amount of food available during scheduled feeding in DMHPpp1r17 neurons also decreased cFos in DMHPpp1r17 neurons. Finally, these neurons do not express the leptin receptor, suggesting that the effect of leptin on these neurons is indirect and secondary to reduced food intake. In aggregate, these results show that PPP1R17 neurons in the DMH are activated by increased food intake and in turn restrict intake to limit overconsumption, suggesting that they function to constrain binges of eating.
Keywords: DMH; body weight; food intake; leptin; metabolism.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Mokdad A. H., et al., Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289, 76–79 (2003). - PubMed
-
- Schwartz M. W., Woods S. C., Porte D. Jr., Seeley R. J., Baskin D. G., Central nervous system control of food intake. Nature 404, 661–671 (2000). - PubMed
-
- Friedman J. M., Halaas J. L., Leptin and the regulation of body weight in mammals. Nature 395, 763–770 (1998). - PubMed
-
- Zhang Y., et al., Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

