Regorafenib enhances antitumor immunity via inhibition of p38 kinase/Creb1/Klf4 axis in tumor-associated macrophages
- PMID: 33753566
- PMCID: PMC7986673
- DOI: 10.1136/jitc-2020-001657
Regorafenib enhances antitumor immunity via inhibition of p38 kinase/Creb1/Klf4 axis in tumor-associated macrophages
Abstract
Background: Regorafenib and other multikinase inhibitors may enhance antitumor efficacy of anti-program cell death-1 (anti-PD1) therapy in hepatocellular carcinoma (HCC). Its immunomodulatory effects, besides anti-angiogenesis, were not clearly defined.
Methods: In vivo antitumor efficacy was tested in multiple syngeneic liver cancer models. Murine bone marrow-derived macrophages (BMDMs) were tested in vitro for modulation of polarization by regorafenib and activation of cocultured T cells. Markers of M1/M2 polarization were measured by quantitative reverse transcription PCR (RT-PCR), arginase activity, flow cytometry, and ELISA. Knockdown of p38 kinase and downstream Creb1/Klf4 signaling on macrophage polarization were confirmed by using knockdown of the upstream MAPK14 kinase, chemical p38 kinase inhibitor, and chromatin immunoprecipitation.
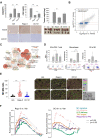
Results: Regorafenib (5 mg/kg/day, corresponding to about half of human clinical dosage) inhibited tumor growth and angiogenesis in vivo similarly to DC-101 (anti-VEGFR2 antibody) but produced higher T cell activation and M1 macrophage polarization, increased the ratio of M1/M2 polarized BMDMs and proliferation/activation of cocultured T cells in vitro, indicating angiogenesis-independent immunomodulatory effects. Suppression of p38 kinase phosphorylation and downstream Creb1/Klf4 activity in BMDMs by regorafenib reversed M2 polarization. Regorafenib enhanced antitumor efficacy of adoptively transferred antigen-specific T cells. Synergistic antitumor efficacy between regorafenib and anti-PD1 was associated with multiple immune-related pathways in the tumor microenvironment.
Conclusion: Regorafenib may enhance antitumor immunity through modulation of macrophage polarization, independent of its anti-angiogenic effects. Optimization of regorafenib dosage for rational design of combination therapy regimen may improve the therapeutic index in the clinic.
Keywords: immunomodulation; immunotherapy; tumor microenvironment.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: Dr A-L Cheng is a consultant for and a member of the speaker’s bureau of Bayer-Schering Pharma. Dr A-L Cheng is a consultant of Novartis, Merck Serono, Eisai, Merck Sharp & Dohme (MSD) Corp., ONXEO, Bayer HealthCare Pharmaceuticals Inc., Bristol-Myers Squibb (BMS) Company, and Ono Pharmaceutical Co., Ltd. Dr C Hsu received research grants from BMS/ONO, Roche, and Ipsen and received honorarium from the following pharmaceutical companies: AstraZeneca, Bayer, BMS/ONO, Eisai, Eli Lilly, Ipsen, Merck Serono, MSD, Novartis, Roche, and TTY Biopharm.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
