Effect of cabazitaxel on macrophages improves CD47-targeted immunotherapy for triple-negative breast cancer
- PMID: 33753567
- PMCID: PMC7986678
- DOI: 10.1136/jitc-2020-002022
Effect of cabazitaxel on macrophages improves CD47-targeted immunotherapy for triple-negative breast cancer
Abstract
Background: Limited therapeutic options are available for triple-negative breast cancer (TNBC), emphasizing an urgent need for more effective treatment approaches. The development of strategies by targeting tumor-associated macrophages (TAMs) to stimulate their ability of Programmed Cell Removal (PrCR) provides a promising new immunotherapy for TNBC treatment.
Methods: CD47 is a critical self-protective "don't eat me" signal on multiple human cancers against macrophage immunosurveillance. Using human and mouse TNBC preclinical models, we evaluated the efficacy of PrCR-based immunotherapy by blocking CD47. We performed high-throughput screens on FDA-approved anti-cancer small molecule compounds for agents potentiating PrCR and enhancing the efficacy of CD47-targeted therapy for TNBC treatment.
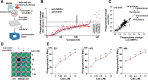
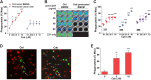
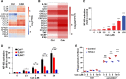
Results: We showed that CD47 was widely expressed on TNBC cells and TAMs represented the most abundant immune cell population in TNBC tumors. Blockade of CD47 enabled PrCR of TNBC cells, but the efficacy was not satisfactory. Our high-throughput screens identified cabazitaxel in enhancing PrCR-based immunotherapy. A combination of CD47 blockade and cabazitaxel treatment yielded a highly effective treatment strategy, promoting PrCR of TNBC cells and inhibiting tumor development and metastasis in preclinical models. We demonstrated that cabazitaxel potentiated PrCR by activating macrophages, independent of its cytotoxicity toward cancer cells. When treated with cabazitaxel, the molecular and phenotypic signatures of macrophages were polarized toward M1 state, and the NF-kB signaling pathway became activated.
Conclusion: The combination of CD47 blockade and macrophage activation by cabazitaxel synergizes to vastly enhance the elimination of TNBC cells. Our results show that targeting macrophages is a promising and effective strategy for TNBC treatment.
Keywords: immunotherapy; macrophages; phagocytosis; triple-negative breast cancer; tumor microenvironment.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MF declares patent applications pertaining to stimulating TLR/BTK signaling to promote CRT in macrophages assigned to the Stanford University and equity and/or consulting with Forty Seven, Inc.
Figures



References
-
- Garrido-Castro AC, Lin NU, Polyak K. Insights into molecular classifications of triple-negative breast cancer: improving patient selection for treatment. Cancer Discov 2019;9:176–98. 10.1158/2159-8290.CD-18-1177 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
