Systemic pro-inflammatory response identifies patients with cancer with adverse outcomes from SARS-CoV-2 infection: the OnCovid Inflammatory Score
- PMID: 33753569
- PMCID: PMC7985977
- DOI: 10.1136/jitc-2020-002277
Systemic pro-inflammatory response identifies patients with cancer with adverse outcomes from SARS-CoV-2 infection: the OnCovid Inflammatory Score
Erratum in
-
Correction: Systemic pro-inflammatory response identifies patients with cancer with adverse outcomes from SARS-CoV-2 infection: the OnCovid Inflammatory Score.J Immunother Cancer. 2021 Jun;9(6):e002277corr1. doi: 10.1136/jitc-2020-002277corr1. J Immunother Cancer. 2021. PMID: 34135105 Free PMC article. No abstract available.
Abstract
Background: Patients with cancer are particularly susceptible to SARS-CoV-2 infection. The systemic inflammatory response is a pathogenic mechanism shared by cancer progression and COVID-19. We investigated systemic inflammation as a driver of severity and mortality from COVID-19, evaluating the prognostic role of commonly used inflammatory indices in SARS-CoV-2-infected patients with cancer accrued to the OnCovid study.
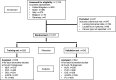
Methods: In a multicenter cohort of SARS-CoV-2-infected patients with cancer in Europe, we evaluated dynamic changes in neutrophil:lymphocyte ratio (NLR); platelet:lymphocyte ratio (PLR); Prognostic Nutritional Index (PNI), renamed the OnCovid Inflammatory Score (OIS); modified Glasgow Prognostic Score (mGPS); and Prognostic Index (PI) in relation to oncological and COVID-19 infection features, testing their prognostic potential in independent training (n=529) and validation (n=542) sets.
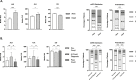
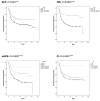
Results: We evaluated 1071 eligible patients, of which 625 (58.3%) were men, and 420 were patients with malignancy in advanced stage (39.2%), most commonly genitourinary (n=216, 20.2%). 844 (78.8%) had ≥1 comorbidity and 754 (70.4%) had ≥1 COVID-19 complication. NLR, OIS, and mGPS worsened at COVID-19 diagnosis compared with pre-COVID-19 measurement (p<0.01), recovering in survivors to pre-COVID-19 levels. Patients in poorer risk categories for each index except the PLR exhibited higher mortality rates (p<0.001) and shorter median overall survival in the training and validation sets (p<0.01). Multivariable analyses revealed the OIS to be most independently predictive of survival (validation set HR 2.48, 95% CI 1.47 to 4.20, p=0.001; adjusted concordance index score 0.611).
Conclusions: Systemic inflammation is a validated prognostic domain in SARS-CoV-2-infected patients with cancer and can be used as a bedside predictor of adverse outcome. Lymphocytopenia and hypoalbuminemia as computed by the OIS are independently predictive of severe COVID-19, supporting their use for risk stratification. Reversal of the COVID-19-induced proinflammatory state is a putative therapeutic strategy in patients with cancer.
Keywords: COVID-19; inflammation; inflammation mediators.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: DJP received lecture fees from ViiV Healthcare and Bayer Healthcare and travel expenses from BMS and Bayer Healthcare; consulting fees for Mina Therapeutics, EISAI, Roche, and Astra Zeneca; research funding (to institution) from MSD and BMS. AP has declared personal honoraria from Pfizer, Novartis, Roche, MSD Oncology, Eli Lilly, and Daiichi Sankyo; travel, accommodations, and expenses paid by Daiichi Sankyo; research funding from Roche and Novartis; and consulting/advisory role for NanoString Technologies, Amgen, Roche, Novartis, Pfizer and Bristol-Myers Squibb. TND has declared consulting/advisory role for Amgen, Bayer, AstraZeneca, BMS, Boehringer Ingelheim, Eli Lilly, MSD, Novartis, Otsuka, Pfizer, Roche, and Takeda; speakers fees from AstraZeneca, MSD, Roche, Takeda; and travel, accommodations and expenses paid by AstraZenca, BMS, Boehringer Ingelheim, Lilly, MSD, Otsuka, Roche, and Takeda. JB has declared consulting/advisory role for MSD and Astra Zeneca. PPS has declared consulting/advisory role for Sanofi and Abbvie. AP has declared consulting/advisory role for Takeda and Sanofi. MP has declared consulting/advisory role for Gilead and Bayer. AG has declared consulting/advisory role for Roche, MSD, Eli Lilly, Pierre Fabre, EISAI, and Daichii Sankyo; speakers bureau for Eisai, Novartis, Eli Lilly, Roche, Teva, Gentili, Pfizer, Astra Zeneca, Celgene, and Daichii Sankyo; research funds: EISAI, Eli Lilly, and Roche. LR reports receiving consulting fees from Amgen, ArQule, AstraZeneca, Basilea, Bayer, Celgene, Eisai, Exelixis, Hengrui, Incyte, Ipsen, Lilly, MSD, Nerviano Medical Sciences, Roche, and Sanofi; lectures fees from AbbVie, Amgen, Eisai, Gilead, Incyte, Ipsen, Lilly, Roche, and Sanofi; travel fees from Ipsen; and institutional research funding from Agios, ARMO BioSciences, AstraZeneca, BeiGene, Eisai, Exelixis, Fibrogen, Incyte, Ipsen, Lilly, MSD, and Roche. All remaining authors have declared no conflicts of interest.
Figures


References
-
- Center for Systems Science and Engineering . Coronavirus COVID-19 global cases, 2019. Available: https://coronavirus.jhu.edu/map.html
-
- Organization WH . Clinical management of COVID-19: interim guidance. V 1.5. 1.5 ED 2020.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
