SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19
- PMID: 33753738
- PMCID: PMC7985370
- DOI: 10.1038/s41467-021-22034-1
SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19
Abstract
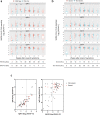
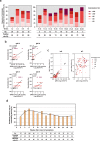
Long-term antibody responses and neutralizing activities in response to SARS-CoV-2 infection are not yet clear. Here we quantify immunoglobulin M (IgM) and G (IgG) antibodies recognizing the SARS-CoV-2 receptor-binding domain (RBD) of the spike (S) or the nucleocapsid (N) protein, and neutralizing antibodies during a period of 6 months from COVID-19 disease onset in 349 symptomatic COVID-19 patients who were among the first be infected world-wide. The positivity rate and magnitude of IgM-S and IgG-N responses increase rapidly. High levels of IgM-S/N and IgG-S/N at 2-3 weeks after disease onset are associated with virus control and IgG-S titers correlate closely with the capacity to neutralize SARS-CoV-2. Although specific IgM-S/N become undetectable 12 weeks after disease onset in most patients, IgG-S/N titers have an intermediate contraction phase, but stabilize at relatively high levels over the 6 month observation period. At late time points, the positivity rates for binding and neutralizing SARS-CoV-2-specific antibodies are still >70%. These data indicate sustained humoral immunity in recovered patients who had symptomatic COVID-19, suggesting prolonged immunity.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- WHO. Coronavirus Disease (COVID-19) Situation Dashboard (WHO, accessed 18 Jan 2021). https://covid19.who.int.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

