Learned spectral decoloring enables photoacoustic oximetry
- PMID: 33753769
- PMCID: PMC7985523
- DOI: 10.1038/s41598-021-83405-8
Learned spectral decoloring enables photoacoustic oximetry
Abstract
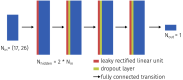
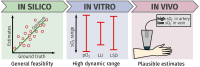
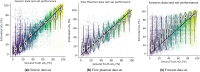
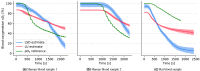
The ability of photoacoustic imaging to measure functional tissue properties, such as blood oxygenation sO[Formula: see text], enables a wide variety of possible applications. sO[Formula: see text] can be computed from the ratio of oxyhemoglobin HbO[Formula: see text] and deoxyhemoglobin Hb, which can be distuinguished by multispectral photoacoustic imaging due to their distinct wavelength-dependent absorption. However, current methods for estimating sO[Formula: see text] yield inaccurate results in realistic settings, due to the unknown and wavelength-dependent influence of the light fluence on the signal. In this work, we propose learned spectral decoloring to enable blood oxygenation measurements to be inferred from multispectral photoacoustic imaging. The method computes sO[Formula: see text] pixel-wise, directly from initial pressure spectra [Formula: see text], which represent initial pressure values at a fixed spatial location [Formula: see text] over all recorded wavelengths [Formula: see text]. The method is compared to linear unmixing approaches, as well as pO[Formula: see text] and blood gas analysis reference measurements. Experimental results suggest that the proposed method is able to obtain sO[Formula: see text] estimates from multispectral photoacoustic measurements in silico, in vitro, and in vivo.
Conflict of interest statement
The authors declare no competing interests.
Figures


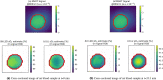
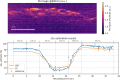
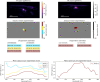
References
-
- Haacke EM, Lai S, Yablonskiy DA, Lin W. In vivo validation of the bold mechanism: A review of signal changes in gradient echo functional mri in the presence of flow. Int. J. Imaging Syst. Technol. 1995;6:153–163. doi: 10.1002/ima.1850060204. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

