Chemotherapy-based versus chemotherapy-free stem cell mobilization (± plerixafor) in multiple myeloma patients: an Italian cost-effectiveness analysis
- PMID: 33753907
- PMCID: PMC8338551
- DOI: 10.1038/s41409-021-01251-8
Chemotherapy-based versus chemotherapy-free stem cell mobilization (± plerixafor) in multiple myeloma patients: an Italian cost-effectiveness analysis
Abstract
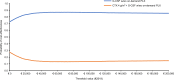
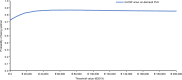
Given the availability and efficacy of the mobilizing agent plerixafor in augmenting hematopoietic progenitor cell mobilization with granulocyte colony-stimulating factor (G-CSF), there is a strong case for comparing the cost-effectiveness of mobilization with G-CSF + cyclophosphamide versus G-CSF alone. This study investigated the cost and effectiveness (i.e., successful 4 million-CD34+ collection) of G-CSF alone versus high-dose cyclophosphamide (4 g/m2) + G-CSF mobilization (± on-demand plerixafor) in patients with multiple myeloma (MM) eligible for autograft in Italy. A decision tree-supported cost-effectiveness analysis (CEA) model in MM patients was developed from the societal perspective. The CEA model compared G-CSF alone with cyclophosphamide 4 g/m2 + G-CSF (± on-demand plerixafor) and was populated with demographic, healthcare and non-healthcare resource utilization data collected from a questionnaire administered to six Italian oncohematologists. Costs were expressed in Euro (€) 2019. The CEA model showed that G-CSF alone was strongly dominant versus cyclophosphamide + G-CSF ( ± on-demand plerixafor), with incremental savings of €1198.59 and an incremental probability of a successful 4 million-CD34+ apheresis (+0.052). Sensitivity analyses confirmed the robustness of the base-case results. In conclusion, chemotherapy-free mobilization (± on-demand plerixafor) is a "good value for money" option for MM patients eligible for autograft.
© 2021. The Author(s).
Conflict of interest statement
CL has received research grants, speaker or consultancy fees from Boehringer Ingelheim Italia S.p.A., CSL Behring S.p.A., Ferring S.p.A., Ipsen S.p.A., Roche S.p.A., Sanofi S.p.A., Santen GmbH, and Shire Italia S.p.A.; LC has received consultancy fees from Sanofi S.p.A.; FL has received consultancy fees from AbbVie s.r.l. and Sanofi S.p.A.; DL has received consultancy fees from Sanofi S.p.A. and Sandoz S.p.A.; GM declares no disclosures; LP has received consultancy fees from Sanofi S.p.A.; RS has received consultancy fees from Sanofi S.p.A.
Figures


References
-
- Alegre A, Tomas JF, Martinez-Chamorro C, Gil-Fernandez JJ, Fernandez-Villalta MJ, Arranz R, et al. Comparison of peripheral blood progenitor cell mobilization in patients with multiple myeloma: high-dose cyclophosphamide plus GM-CSF vs G-CSF alone. Bone Marrow Transpl. 1997;20:211–7. doi: 10.1038/sj.bmt.1700867. - DOI - PubMed
-
- Goldschmidt H, Hegenbart U, Wallmeier M, Hohaus S, Haas R. Factors influencing collection of peripheral blood progenitor cells following high-dose cyclophosphamide and granulocyte colony-stimulating factor in patients with multiple myeloma. Br J Haematol. 1997;98:736–44. doi: 10.1046/j.1365-2141.1997.2783095.x. - DOI - PubMed
-
- Mohty M, Hubel K, Kroger N, Aljurf M, Apperley J, Basak GW, et al. Autologous haematopoietic stem cell mobilisation in multiple myeloma and lymphoma patients: a position statement from the European Group for Blood and Marrow Transplantation. Bone Marrow Transpl. 2014;49:865–72. doi: 10.1038/bmt.2014.39. - DOI - PubMed
-
- Petrucci MT, Avvisati G, La Verde G, De Fabritiis P, Ribersani M, Palumbo G, et al. Intermediate-dose cyclophosphamide and granulocyte colony-stimulating factor is a valid alternative to high-dose cyclophosphamide for mobilizing peripheral blood CD34+ cells in patients with multiple myeloma. Acta Haematol. 2003;109:184–8. doi: 10.1159/000070967. - DOI - PubMed
-
- Hamadani M, Kochuparambil ST, Osman S, Cumpston A, Leadmon S, Bunner P, et al. Intermediate-dose versus low-dose cyclophosphamide and granulocyte colony-stimulating factor for peripheral blood stem cell mobilization in patients with multiple myeloma treated with novel induction therapies. Biol Blood Marrow Transpl. 2012;18:1128–35. doi: 10.1016/j.bbmt.2012.01.005. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

