Anlotinib suppresses metastasis and multidrug resistance via dual blockade of MET/ABCB1 in colorectal carcinoma cells
- PMID: 33754008
- PMCID: PMC7974540
- DOI: 10.7150/jca.45618
Anlotinib suppresses metastasis and multidrug resistance via dual blockade of MET/ABCB1 in colorectal carcinoma cells
Abstract
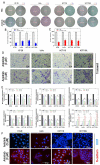
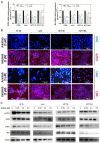
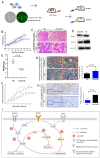
Anlotinib, a highly selective multi-targeted tyrosine kinase inhibitor (TKI) has therapeutic effects on non-small-cell lung cancer (NSCLC). In this study, the anti-tumor activity and molecular mechanism of anlotinib in metastatic colorectal cancer (mCRC) was explored. The anti-angiogenesis, anti-metastasis, anti-proliferative, and anti-multidrug resistance efficacy of anlotinib were analyzed by using in vitro and in vivo models of human CRC cells. The results indicated that anlotinib boosted chemo-sensitivity of CRC cells, and restrained its proliferation. Besides the suppression of the MET signaling pathway, anlotinib also inhibited invasion and migration of CRC cells. Furthermore, anlotinib prevented VEGF-induced angiogenesis, N-cadherin (CDH2)-induced cell migration, and reversed ATP-binding cassette subfamily B member 1 (ABCB1) -mediated CRC multidrug resistance in CRC. The CRC liver metastasis and subcutaneously implanted xenograft model testified that anlotinib could inhibit proliferation and liver metastasis in CRC cells. Such an observation suggested that a combination of anlotinib with anti-cancer drugs could attenuate angiogenesis, metastasis, proliferative, and multidrug resistance, which constitutes a novel treatment strategy for CRC patients with metastasis.
Keywords: ABCB1; MET; anlotinb; colorectal carcinoma; metastasis.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
Anlotinib, a novel small molecular tyrosine kinase inhibitor, suppresses growth and metastasis via dual blockade of VEGFR2 and MET in osteosarcoma.Int J Cancer. 2019 Aug 15;145(4):979-993. doi: 10.1002/ijc.32180. Epub 2019 Feb 15. Int J Cancer. 2019. PMID: 30719715
-
Anlotinib Suppresses Colorectal Cancer Proliferation and Angiogenesis via Inhibition of AKT/ERK Signaling Cascade.Cancer Manag Res. 2020 Jun 24;12:4937-4948. doi: 10.2147/CMAR.S252181. eCollection 2020. Cancer Manag Res. 2020. PMID: 32606981 Free PMC article.
-
Anlotinib Reverses Multidrug Resistance (MDR) in Osteosarcoma by Inhibiting P-Glycoprotein (PGP1) Function In Vitro and In Vivo.Front Pharmacol. 2022 Jan 17;12:798837. doi: 10.3389/fphar.2021.798837. eCollection 2021. Front Pharmacol. 2022. PMID: 35111065 Free PMC article.
-
Anlotinib: A Novel Targeted Drug for Bone and Soft Tissue Sarcoma.Front Oncol. 2021 May 20;11:664853. doi: 10.3389/fonc.2021.664853. eCollection 2021. Front Oncol. 2021. PMID: 34094958 Free PMC article. Review.
-
Effective treatment of anlotinib in giant delayed pulmonary metastasis of osteosarcoma: a case report and literature review.Ann Palliat Med. 2021 Jun;10(6):7073-7082. doi: 10.21037/apm-20-1790. Epub 2021 Jan 29. Ann Palliat Med. 2021. PMID: 33548989 Review.
Cited by
-
Downregulation of LncRNA SNHG7 Sensitizes Colorectal Cancer Cells to Resist Anlotinib by Regulating miR-181a-5p/GATA6.Gastroenterol Res Pract. 2023 Jan 14;2023:6973723. doi: 10.1155/2023/6973723. eCollection 2023. Gastroenterol Res Pract. 2023. PMID: 36691432 Free PMC article.
-
Anlotinib Suppressed Ovarian Cancer Progression via Inducing G2/M Phase Arrest and Apoptosis.J Clin Med. 2022 Dec 25;12(1):162. doi: 10.3390/jcm12010162. J Clin Med. 2022. PMID: 36614964 Free PMC article.
-
LncRNA SNHG16 promotes colorectal cancer proliferation by regulating ABCB1 expression through sponging miR-214-3p.J Biomed Res. 2022 Jun 28;36(4):231-241. doi: 10.7555/JBR.36.20220049. J Biomed Res. 2022. PMID: 35965433 Free PMC article.
-
Anlotinib Inhibits Cisplatin Resistance in Non-Small-Cell Lung Cancer Cells by Inhibiting MCL-1 Expression via MET/STAT3/Akt Pathway.Can Respir J. 2024 Mar 4;2024:2632014. doi: 10.1155/2024/2632014. eCollection 2024. Can Respir J. 2024. PMID: 38468814 Free PMC article.
References
-
- Torre LA, Bray F, Siegel RL. et al. Global cancer statistics. 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Siegel R, DeSantis C, Virgo K. et al. Cancer treatment and survivorship statistics. 2012. CA Cancer J Clin. 2012;62:220–241. - PubMed
-
- Michl M, Thurmaier J, Schubert-Fritschle G. et al. Brain Metastasis in Colorectal Cancer Patients: Survival and Analysis of Prognostic Factors. Clin Colorectal Cancer. 2015;14:281–290. - PubMed
-
- Sun Y, Wu X, Zhang Y. et al. Pathological complete response may underestimate distant metastasis in locally advanced rectal cancer following neoadjuvant chemoradiotherapy and radical surgery: Incidence, metastatic pattern, and risk factors. Eur J Surg Oncol. 2019;45:1225–1231. - PubMed
-
- Weng W, Feng J, Qin H. et al. Molecular therapy of colorectal cancer: progress and future directions. Int J Cancer. 2015;136:493–502. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

