The double-edged roles of ROS in cancer prevention and therapy
- PMID: 33754031
- PMCID: PMC7978298
- DOI: 10.7150/thno.56747
The double-edged roles of ROS in cancer prevention and therapy
Abstract
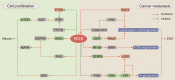
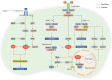
Reactive oxygen species (ROS) serve as cell signaling molecules generated in oxidative metabolism and are associated with a number of human diseases. The reprogramming of redox metabolism induces abnormal accumulation of ROS in cancer cells. It has been widely accepted that ROS play opposite roles in tumor growth, metastasis and apoptosis according to their different distributions, concentrations and durations in specific subcellular structures. These double-edged roles in cancer progression include the ROS-dependent malignant transformation and the oxidative stress-induced cell death. In this review, we summarize the notable literatures on ROS generation and scavenging, and discuss the related signal transduction networks and corresponding anticancer therapies. There is no doubt that an improved understanding of the sophisticated mechanism of redox biology is imperative to conquer cancer.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

