FOSL2 promotes VEGF-independent angiogenesis by transcriptionnally activating Wnt5a in breast cancer-associated fibroblasts
- PMID: 33754039
- PMCID: PMC7978317
- DOI: 10.7150/thno.55074
FOSL2 promotes VEGF-independent angiogenesis by transcriptionnally activating Wnt5a in breast cancer-associated fibroblasts
Erratum in
-
Erratum: FOSL2 promotes VEGF-independent angiogenesis by transcriptionnally activating Wnt5a in breast cancer-associated fibroblasts: Erratum.Theranostics. 2022 Aug 18;12(14):6157-6158. doi: 10.7150/thno.77019. eCollection 2022. Theranostics. 2022. PMID: 36168614 Free PMC article.
Abstract
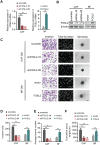
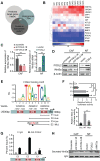
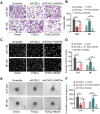
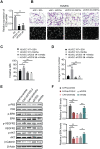
Cancer-associated fibroblasts (CAFs), a predominant component of the tumor microenvironment, contribute to aggressive angiogenesis progression. In clinical practice, traditional anti-angiogenic therapy, mainly anti-VEGF, provides extremely limited beneficial effects to breast cancer. Here, we reveal that FOS-like 2 (FOSL2), a transcription factor in breast CAFs, plays a critical role in VEGF-independent angiogenesis in stromal fibroblasts. Methods: FOSL2 and Wnt5a expression was assessed by qRT-PCR, western blotting and immunohistochemistry in primary and immortalized CAFs and clinical samples. FOSL2- or Wnt5a-silenced CAFs and FOSL2-overexpressing NFs were established to explore their proangiogenic effects. Invasion, tubule formation, three-dimensional sprouting assays, and orthotopic xenografts were conducted as angiogenesis experiments. FZD5/NF-κB/ERK signaling activation was evaluated by western blotting after blocking VEGF/VEGFR with an anti-VEGF antibody and axitinib. Dual luciferase reporter assays and chromatin immunoprecipitation were performed to test the role of FOSL2 in regulating Wnt5a expression, and Wnt5a in the serum of the patients was measured to assess its clinical diagnostic value for breast cancer patients. Results: Enhanced FOSL2 in breast CAFs was significantly associated with angiogenesis and clinical progression in patients. The supernatant from CAFs highly expressing FOSL2 strongly promoted tube formation and sprouting of human umbilical vein endothelial cells (HUVECs) in a VEGF-independent manner and angiogenesis as well as tumor growth in vivo. Mechanistically, the enhanced FOSL2 in CAFs was regulated by estrogen/cAMP/PKA signaling. Wnt5a, a direct target of FOSL2, specifically activated FZD5/NF-κB/ERK signaling in HUVECs to promote VEGF-independent angiogenesis. In addition, a high level of Wnt5a was commonly detected in the serum of breast cancer patients and closely correlated with microvessel density in breast tumor tissues, suggesting a promising clinical value of Wnt5a for breast cancer diagnostics. Conclusion: FOSL2/Wnt5a signaling plays an essential role in breast cancer angiogenesis in a VEGF-independent manner, and targeting the FOSL2/Wnt5a signaling axis in CAFs may offer a potential option for antiangiogenesis therapy.
Keywords: FOSL2; VEGF-independent angiogenesis; Wnt5a; cancer-associated fibroblasts.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

