NAD+-boosting therapy alleviates nonalcoholic fatty liver disease via stimulating a novel exerkine Fndc5/irisin
- PMID: 33754067
- PMCID: PMC7977447
- DOI: 10.7150/thno.53652
NAD+-boosting therapy alleviates nonalcoholic fatty liver disease via stimulating a novel exerkine Fndc5/irisin
Abstract
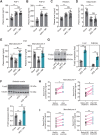
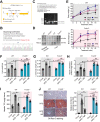
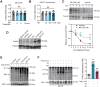
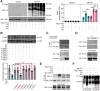
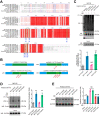
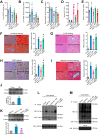
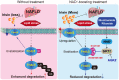
Rationale: Nicotinamide adenine dinucleotide+ (NAD+)-boosting therapy has emerged as a promising strategy to treat various health disorders, while the underlying molecular mechanisms are not fully understood. Here, we investigated the involvement of fibronectin type III domain containing 5 (Fndc5) or irisin, which is a novel exercise-linked hormone, in the development and progression of nonalcoholic fatty liver disease (NAFLD). Methods: NAD+-boosting therapy was achieved by administrating of nicotinamide riboside (NR) in human and mice. The Fndc5/irisin levels in tissues and blood were measured in NR-treated mice or human volunteers. The therapeutic action of NR against NAFLD pathologies induced by high-fat diet (HFD) or methionine/choline-deficient diet (MCD) were compared between wild-type (WT) and Fndc5-/- mice. Recombinant Fndc5/irisin was infused to NALFD mice via osmotic minipump to test the therapeutic action of Fndc5/irisin. Various biomedical experiments were conducted in vivo and in vitro to know the molecular mechanisms underlying the stimulation of Fndc5/irisin by NR treatment. Results: NR treatment elevated plasma level of Fndc5/irisin in mice and human volunteers. NR treatment also increased Fndc5 expression in skeletal muscle, adipose and liver tissues in mice. In HFD-induced NAFLD mice model, NR displayed remarkable therapeutic effects on body weight gain, hepatic steatosis, steatohepatitis, insulin resistance, mitochondrial dysfunction, apoptosis and fibrosis; however, these actions of NR were compromised in Fndc5-/- mice. Chronic infusion of recombinant Fndc5/irisin alleviated the NAFLD pathological phenotypes in MCD-induced NAFLD mice model. Mechanistically, NR reduced the lipid stress-triggered ubiquitination of Fndc5, which increased Fndc5 protein stability and thus enhanced Fndc5 protein level. Using shRNA-mediated knockdown screening, we found that NAD+-dependent deacetylase SIRT2, rather than other sirtuins, interacts with Fndc5 to decrease Fndc5 acetylation, which reduces Fndc5 ubiquitination and stabilize it. Treatment of AGK2, a selective inhibitor of SIRT2, blocked the therapeutic action of NR against NAFLD pathologies and NR-induced Fndc5 deubiquitination/deacetylation. At last, we identified that the lysine sites K127/131 and K185/187/189 of Fndc5 may contribute to the SIRT2-dependent deacetylation and deubiquitination of Fndc5. Conclusions: The findings from this research for the first time demonstrate that NAD+-boosting therapy reverses NAFLD by regulating SIRT2-deppendent Fndc5 deacetylation and deubiquitination, which results in a stimulation of Fndc5/irisin, a novel exerkine. These results suggest that Fndc5/irisin may be a potential nexus between physical exercise and NAD+-boosting therapy in metabolic pathophysiology.
Keywords: Fndc5; NAD+; SIRT2; irisin; nonalcoholic fatty liver disease; physical exercise.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

