Pancreatic ductal deletion of S100A9 alleviates acute pancreatitis by targeting VNN1-mediated ROS release to inhibit NLRP3 activation
- PMID: 33754072
- PMCID: PMC7977474
- DOI: 10.7150/thno.54245
Pancreatic ductal deletion of S100A9 alleviates acute pancreatitis by targeting VNN1-mediated ROS release to inhibit NLRP3 activation
Abstract
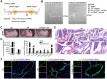
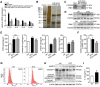
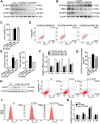
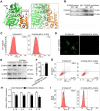
Recent studies have proven that the overall pathophysiology of pancreatitis involves not only the pancreatic acinar cells but also duct cells, however, pancreatic duct contribution in acinar cells homeostasis is poorly known and the molecular mechanisms leading to acinar insult and acute pancreatitis (AP) are unclear. Our previous work also showed that S100A9 protein level was notably increased in AP rat pancreas through iTRAQ-based quantitative proteomic analysis. Therefore, we investigated the actions of injured duct cells on acinar cells and the S100A9-related effects and mechanisms underlying AP pathology in the present paper. Methods: In this study, we constructed S100A9 knockout (s100a9-/-) mice and an in vitro coculture system for pancreatic duct cells and acinar cells. Moreover, a variety of small molecular inhibitors of S100A9 were screened from ChemDiv through molecular docking and virtual screening methods. Results: We found that the upregulation of S100A9 induces cell injury and inflammatory response via NLRP3 activation by targeting VNN1-mediated ROS release; and loss of S100A9 decreases AP injury in vitro and in vivo. Moreover, molecular docking and mutant plasmid experiments proved that S100A9 has a direct interaction with VNN1 through the salt bridges formation of Lys57 and Glu92 residues in S100A9 protein. We further found that compounds C42H60N4O6 and C28H29F3N4O5S can significantly improve AP injury in vitro and in vivo through inhibiting S100A9-VNN1 interaction. Conclusions: Our study showed the important regulatory effect of S100A9 on pancreatic duct injury during AP and revealed that inhibition of the S100A9-VNN1 interaction may be a key therapeutic target for this disease.
Keywords: S100A9; VNN1; acinar cells; acute pancreatitis; duct cells.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- van Dijk SM, Hallensleben NDL, van Santvoort HC, Fockens P, van Goor H, Bruno MJ. et al. Acute pancreatitis: recent advances through randomised trials. Gut. 2017;66:2024–32. - PubMed
-
- Tenner S, Baillie J, DeWitt J, Vege SS, American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–15. 1416. - PubMed
-
- Lankisch PG, Ape M, Banks PA, Acute pancreatitis. Lancet. 2015; 386: 85-96. - PubMed
-
- Trikudanathan G, Wolbrink DRJ, van Santvoort HC, Mallery S, Freeman M, Besselink MG. Current concepts in severe acute and necrotizing pancreatitis: an evidence-based approach. Gastroenterology. 2019;156:1994–2007.e3. - PubMed
-
- Hines QJ, Pandol SJ. Management of severe acute pancreatitis. BMJ. 2019;367:l6227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

