Highly Purified Cannabidiol for Epilepsy Treatment: A Systematic Review of Epileptic Conditions Beyond Dravet Syndrome and Lennox-Gastaut Syndrome
- PMID: 33754312
- PMCID: PMC8005394
- DOI: 10.1007/s40263-021-00807-y
Highly Purified Cannabidiol for Epilepsy Treatment: A Systematic Review of Epileptic Conditions Beyond Dravet Syndrome and Lennox-Gastaut Syndrome
Abstract
Background: Cannabidiol (CBD), which is one major constituent of the Cannabis sativa plant, has anti-seizure properties and does not produce euphoric or intrusive side effects. A plant-derived, highly purified CBD formulation with a known and constant composition has been approved by the US Food and Drug Administration for the treatment of seizures associated with Dravet syndrome, Lennox-Gastaut syndrome, and tuberous sclerosis complex. In the European Union, the drug has been authorized by the European Medicines Agency for the treatment of seizures associated with Dravet syndrome and Lennox-Gastaut syndrome, in conjunction with clobazam, and is under regulatory review for the treatment of seizures in patients with tuberous sclerosis complex.
Objectives: This systematic review aimed to summarize the currently available body of knowledge about the use of this US Food and Drug Administration/European Medicines Agency-approved oral formulation of pharmaceutical-grade CBD in patients with epileptic conditions, especially developmental and epileptic encephalopathies other than Dravet syndrome and Lennox-Gastaut syndrome.
Methods: The relevant studies were identified through MEDLINE and the US National Institutes of Health Clinical Trials Registry in October 2020. There were no date limitations or language restrictions. The following types of studies were included: clinical trials, cohorts, case-control, cross-sectional, clinical series, and case reports. Participants had to meet the following criteria: any sex, any ethnicity, any age, diagnosis of epilepsy, receiving plant-derived, highly purified (> 98% w/w) CBD in a sesame oil-based oral solution for the treatment of seizures. Data extracted from selected records included efficacy, tolerability, and safety outcomes.
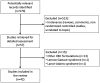
Results: Five hundred and seventy records were identified by database and trial register searching. Fifty-seven studies were retrieved for detailed assessment, of which 42 were eventually included for the review. The participants of the studies included patients of both pediatric and adult age. Across the trials, purified CBD was administered at dosages up to 50 mg/kg/day. In a randomized double-blind controlled trial in patients with tuberous sclerosis complex, CBD was associated with a significantly greater percent reduction in seizure frequency than placebo over the treatment period. Open-label studies suggested the effectiveness of CBD in the treatment of children and adults presenting with other epilepsy syndromes than those addressed by regulatory trials, including CDKL5 deficiency disorder and Aicardi, Dup15q, and Doose syndromes, SYNGAP1 encephalopathy, and epilepsy with myoclonic absences. The most common adverse events observed during treatment with CBD included somnolence, decreased appetite, diarrhea, and increased serum aminotransferases.
Conclusions: The currently available data suggest that response to treatment with a highly purified, plant-derived CBD oil-based solution can be seen in patients across a broad range of epilepsy disorders and etiologies. The existing evidence can provide preliminary support for additional research.
Conflict of interest statement
Simona Lattanzi has received speaker’s or consultancy fees from Eisai, UCB Pharma, and GW Pharmaceuticals and has served on advisory boards for Angelini Pharma, Arvelle Therapeutics, BIAL, and GW Pharmaceuticals. Eugen Trinka has received speaker’s honoraria from UCB Pharma, Biogen, Gerot-Lannach, Bial, Eisai, Takeda, Newbridge, Sunovion Pharmaceuticals Inc., LivaNova, and Novartis; consultancy funds from UCB Pharma, Biogen, Gerot-Lannach, Bial, Eisai, Takeda, Newbridge, GW Pharmaceuticals, Sunovion Pharmaceuticals Inc., and Novartis; and directorship funds from Neuroconsult GmbH. Eugen Trinka’s institution received grants from Biogen, Red Bull, Merck, UCB Pharma, European Union, FWF Österreichischer Fond zur Wissenschaftsförderung, and Bundesministerium für Wissenschaft und Forschung. Pasquale Striano received fees and research grants from GW Pharmaceuticals, Zogenyx, Biomarin, and Kolfarma s.r.l. Chiara Rocchi, Sergio Salvemini, and Mauro Silvestrini have no conflicts of interest that are directly relevant to the content of this study. Francesco Brigo acted as a consultant for Eisai.
Figures
Similar articles
-
Real-world experience of cannabidiol in conjunction with clobazam for the treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome: Results from a retrospective multicentre chart review in Germany.Epilepsy Behav. 2025 May;166:110302. doi: 10.1016/j.yebeh.2025.110302. Epub 2025 Mar 11. Epilepsy Behav. 2025. PMID: 40073826
-
Cannabis for the Treatment of Epilepsy: an Update.Curr Neurol Neurosci Rep. 2018 Sep 8;18(11):73. doi: 10.1007/s11910-018-0882-y. Curr Neurol Neurosci Rep. 2018. PMID: 30194563 Review.
-
Real-world evidence on the use of cannabidiol for the treatment of drug resistant epilepsy not related to Lennox-Gastaut syndrome, Dravet syndrome or Tuberous Sclerosis Complex.Seizure. 2023 Nov;112:72-76. doi: 10.1016/j.seizure.2023.09.015. Epub 2023 Sep 17. Seizure. 2023. PMID: 37769547
-
Don't Fear the Reefer-Evidence Mounts for Plant-Based Cannabidiol as Treatment for Epilepsy.Epilepsy Curr. 2019 Mar-Apr;19(2):93-95. doi: 10.1177/1535759719835671. Epilepsy Curr. 2019. PMID: 30955420 Free PMC article.
-
Efficacy and Safety of Cannabidiol in Epilepsy: A Systematic Review and Meta-Analysis.Drugs. 2018 Nov;78(17):1791-1804. doi: 10.1007/s40265-018-0992-5. Drugs. 2018. PMID: 30390221
Cited by
-
Clinical Outcome Data of Children Treated with Cannabis-Based Medicinal Products for Treatment Resistant Epilepsy-Analysis from the UK Medical Cannabis Registry.Neuropediatrics. 2023 Jun;54(3):174-181. doi: 10.1055/a-2002-2119. Epub 2022 Dec 20. Neuropediatrics. 2023. PMID: 36539215 Free PMC article.
-
Case report: Alexander's disease with "head drop" as the main symptom and literature review.Front Neurol. 2022 Dec 19;13:1002527. doi: 10.3389/fneur.2022.1002527. eCollection 2022. Front Neurol. 2022. PMID: 36601294 Free PMC article.
-
[Judicialization of cannabidiol-based products in Brazil: an analysis from 2019 to 2022].Cad Saude Publica. 2023 Oct 9;39(8):e00024723. doi: 10.1590/0102-311XPT024723. eCollection 2023. Cad Saude Publica. 2023. PMID: 37820230 Free PMC article. Portuguese.
-
Refining management strategies for Lennox-Gastaut syndrome: Updated algorithms and practical approaches.Epilepsia Open. 2025 Feb;10(1):85-106. doi: 10.1002/epi4.13075. Epub 2024 Dec 19. Epilepsia Open. 2025. PMID: 39700524 Free PMC article.
-
The Effectiveness and Safety of Cannabidiol in Non-seizure-related Indications: A Systematic Review of Published Randomized Clinical Trials.Pharmaceut Med. 2022 Dec;36(6):353-385. doi: 10.1007/s40290-022-00446-8. Epub 2022 Oct 21. Pharmaceut Med. 2022. PMID: 36271316 Free PMC article.
References
-
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68:326–337. - PubMed
-
- Cagnetti C, Lattanzi S, Foschi N, Provinciali L, Silvestrini M. Seizure course during pregnancy in catamenial epilepsy. Neurology. 2014;83:339–344. - PubMed
-
- Lattanzi S, Zaccara G, Giovannelli F, Grillo E, Nardone R, Silvestrini M, et al. Antiepileptic monotherapy in newly diagnosed focal epilepsy: a network meta-analysis. Acta Neurol Scand. 2019;139:33–41. - PubMed
-
- Lattanzi S, Cagnetti C, Foschi N, Provinciali L, Silvestrini M. Lacosamide monotherapy for partial onset seizures. Seizure. 2015;27:71–74. - PubMed
-
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases