Can mRNA Vaccines Turn the Tables During the COVID-19 Pandemic? Current Status and Challenges
- PMID: 33754328
- PMCID: PMC7985228
- DOI: 10.1007/s40261-021-01022-9
Can mRNA Vaccines Turn the Tables During the COVID-19 Pandemic? Current Status and Challenges
Erratum in
-
Correction to: Can mRNA Vaccines Turn the Tables During the COVID-19 Pandemic? Current Status and Challenges.Clin Drug Investig. 2021 Jun;41(6):511. doi: 10.1007/s40261-021-01053-2. Clin Drug Investig. 2021. PMID: 34089504 Free PMC article. No abstract available.
Abstract
The COVID-19 pandemic continues to affect millions of people across the world. The current global statistics for the disease are 111 million cases and 2.45 million deaths, with new cases emerging each day. Although several drugs including remdesivir have been approved for emergency use, they remain ineffective in bringing the infection under control. Therefore, there is a need for highly effective and safe vaccines against COVID-19. The recent advancements in mRNA vaccines have catapulted them to be forefront in the race to develop vaccines for COVID-19. Two mRNA vaccines, BNT162b2 and mRNA-1273, developed by Pfizer-BioNTech and Moderna Therapeutics, respectively, have been granted authorization for emergency use by the US Food and Drug Administration. Interim analysis of the clinical trials for BNT162b2 and mRNA-1273 vaccines reported an efficacy of 95% and 94.1%, respectively, after the second dose. The adverse events for both the vaccines have been found to be mild to moderate, with mostly injection-site reactions and fatigue. No serious adverse events have been reported. Moreover, Pfizer-BioNTech and Moderna Therapeutics have announced that their vaccines are effective even against the new strains (B.1.17 and B.1.351) of the virus. Both companies are now scaling up the production of the vaccines to meet the global demand. Although the long-term efficacy, safety, and immunogenicity of these vaccines is uncertain, there is hope that they can turn the tables against COVID-19 in this current pandemic situation.
Conflict of interest statement
The authors do not have any conflict/competing interests.
Figures
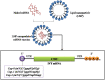
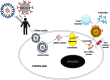
References
-
- COVID-19 Map—Johns Hopkins Coronavirus Resource Center. Johns Hopkins Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu/map.html. Accessed 14 Dec 2020.
-
- Which countries in Europe are experiencing a second wave of COVID-19? [Internet]. euronews. 2020. https://www.euronews.com/2020/11/18/is-europe-having-a-covid-19-second-w.... Accessed 14 Dec 2020.
-
- Coronavirus Second Wave? Why Cases Increase. 2021. https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavir.... Accessed 11 Feb 2021.
-
- Coronavirus disease (COVID-19) advice for the public: When and how to use masks. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-f.... Accessed 14 Dec 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

